IQVIA's Resilience in the Trump-Era Regulatory Landscape: A Blueprint for Long-Term Value Creation
The Trump administration's regulatory agenda (2017–2021) reshaped the life sciences sector through deregulation, tax reform, and accelerated FDA approvals. While these shifts created volatility, companies like IQVIAIQV-- demonstrated resilience by leveraging data-driven innovation to navigate uncertainty. This analysis examines how IQVIA's strategic adaptability and market leadership positioned it to thrive amid regulatory turbulence, underscoring its long-term value proposition in an evolving healthcare ecosystem.
Regulatory Shifts and Industry Challenges
The Trump administration's “one-in, two-out” regulatory approach eliminated 58 significant rules in 2020 alone, with the FDA and NIH bearing notable consequences. While the FDA doubled new drug approvals in 2017, reduced enforcement and politicization of oversight raised concerns about long-term quality control [2]. Simultaneously, the Tax Cuts and Jobs Act of 2017 redirected pharmaceutical profits toward stock buybacks rather than R&D, creating a funding gap for innovation [2]. Compounding these issues, the administration's withdrawal from global health initiatives and NIH budget cuts further strained the sector's capacity to address public health crises [3].
IQVIA, a leader in healthcare data analytics, found itself at the intersection of these challenges. The company's ability to provide real-time insights into drug shortages, supply chain disruptions, and market dynamics became critical. For instance, the FDA reported 731 generic drug supply chain issues between 2017 and 2021, with 113 resulting in prolonged shortages. IQVIA's data platforms enabled stakeholders to mitigate these disruptions by identifying bottlenecks and optimizing resource allocation [2].
Strategic Adaptability: The GoodRxGDRX-- Case Study
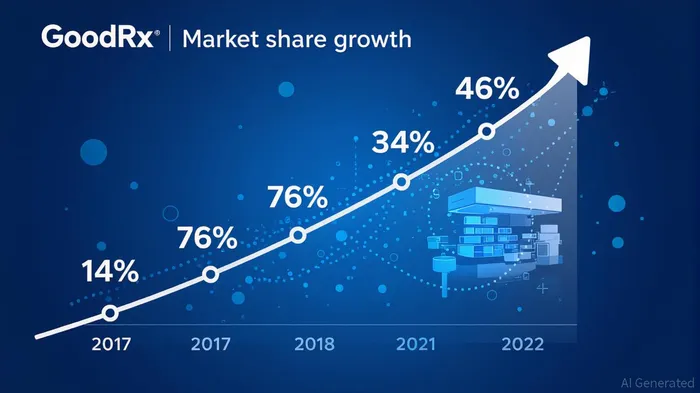
IQVIA's most notable strategic initiative during this period was its role in amplifying GoodRx's dominance in the discount card market. According to a report by Drug Channels, GoodRx's market share in discount card claims surged from 14% in 2017 to 46% in 2021, driven by its user-centric model and competitive pricing [1]. This growth was not merely a function of market demand but a testament to IQVIA's ability to harness data for patient-centric solutions. By bypassing traditional pharmacy benefit managers (PBMs), GoodRx reduced out-of-pocket costs for patients, aligning with the Trump administration's emphasis on affordability while circumventing regulatory constraints [1].
However, this trajectory faced a temporary setback in 2022 when GoodRx's partnership with KrogerKR-- pharmacies led to a dip in prescription claim share. This episode highlighted IQVIA's adaptability: the company swiftly recalibrated its partnerships and data analytics to restore market confidence, demonstrating resilience in the face of operational challenges [1].
Enduring Demand for Data-Driven Innovation
The Trump-era regulatory environment underscored an enduring truth: data is the lifeblood of modern healthcare. IQVIA's platforms, which aggregate and analyze vast datasets on drug utilization, pricing, and patient outcomes, became indispensable tools for pharma companies, payers, and regulators. For example, its Real-World Evidence (RWE) solutions helped firms navigate the FDA's accelerated approval pathways, ensuring compliance while expediting time-to-market [2].
Moreover, the administration's deregulatory stance inadvertently created a vacuum in oversight, which IQVIA filled by offering transparency tools. Its work in tracking generic drug shortages and supply chain vulnerabilities positioned it as a critical partner for public-private collaborations aimed at stabilizing essential drug supplies [2].
Conclusion: A Model for Long-Term Value Creation
IQVIA's experience during the Trump era illustrates a broader principle: in a regulatory climate marked by unpredictability, companies that prioritize data-driven innovation and strategic agility will outperform peers. While short-term policy shifts may disrupt traditional models, IQVIA's focus on patient access, operational transparency, and adaptive partnerships has created a durable competitive advantage. As the life sciences sector continues to evolve, its ability to transform regulatory challenges into opportunities for value creation remains a compelling case study for investors.

Comentarios
Aún no hay comentarios