Investor Protection and Corporate Accountability in Ophthalmic Tech: The RxSight Case Study
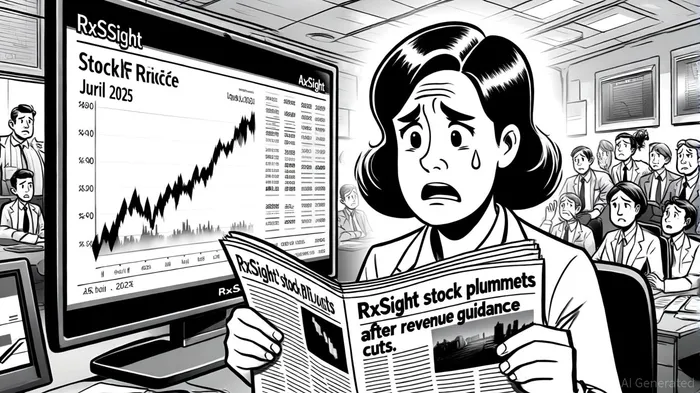
The ongoing legal saga involving RxSightRXST--, Inc. (NASDAQ: RXST) offers a stark illustration of the interplay between investor protection, corporate accountability, and regulatory scrutiny in the ophthalmic technology sector. As the firm faces a securities class action lawsuit led by Faruqi & Faruqi, LLP, the case underscores the risks inherent in balancing innovation with transparency—and the consequences when that balance falters.
The RxSight Case: A Tale of Misrepresentation and Market Reckoning
Faruqi & Faruqi alleges that RxSight and its executives violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 by overstating demand for its flagship products—the Light Delivery Device (LDD) and Light Adjustable Lens (LAL)—and failing to disclose operational challenges that undermined sales performance[1]. These alleged misrepresentations culminated in two major revenue forecast cuts in 2025: first on April 3, 2025, and again on July 8, 2025, when the company reduced its full-year guidance by $42.5 million[2]. The latter announcement triggered a 37.8% stock price plunge, erasing nearly $4.84 in value per share[3].
The lawsuit, Makaveev v. RxSight, Inc., No. 25-cv-01596, highlights a critical issue in investor protection: the duty of companies to disclose material risks that could affect financial performance. According to a Harvard Law School study, firms facing securities fraud lawsuits typically experience a 12.3% abnormal return slump within 20 days of filing, a pattern mirrored by RxSight's stock movements[4]. The case also raises questions about executive accountability, as CEO Ronald Kurtz admitted to adoption challenges only after the market had already priced in the company's failure.
Regulatory Frameworks: FDA and MDR in Ophthalmic Tech
The ophthalmic technology sector operates under a dual regulatory regime. In the U.S., the Food and Drug Administration (FDA) governs device approvals, requiring rigorous pre-market testing for safety and efficacy[5]. However, post-market oversight—particularly for combination products (e.g., devices paired with drugs)—has evolved significantly since 2020. The FDA's updated guidance, following the Genus Medical Technologies court decision, now classifies ophthalmic dispensers as devices, subjecting them to 21 CFR Part 4 and current good manufacturing practice (cGMP) regulations[5].
Meanwhile, the European Medical Device Regulation (MDR), which replaced the Medical Device Directive in 2021, imposes a lifecycle approach to device regulation, emphasizing post-market surveillance and updated clinical evaluations[5]. This divergence creates compliance challenges for global firms like RxSight, which must navigate distinct documentation, quality assurance, and risk-mitigation requirements in the U.S. and Europe.
Investor Protection and the Innovation Dilemma
The RxSight case also intersects with broader debates about investor protection and its impact on innovation. Research suggests that stringent investor protection laws can deter corporate innovation, particularly in high-tech sectors where proprietary information disclosure is costly[6]. For instance, China's 2019 Securities Law revision, which mandated detailed innovation disclosures, led to a 47% decline in corporate patent applications due to litigation risks and competitive exposure[6].
In ophthalmic tech, this tension is amplified by the need to comply with both regulatory and investor protection frameworks. Companies must balance the imperative to innovate with the risks of litigation tied to information asymmetry. RxSight's alleged failure to disclose adoption challenges—despite FDA and MDR requirements—exemplifies how missteps in this balance can lead to market distrust and legal repercussions.
Conclusion: Lessons for Investors and Corporations
The RxSight litigation serves as a cautionary tale for investors and corporate leaders alike. For investors, it underscores the importance of scrutinizing management disclosures, particularly in sectors with high regulatory complexity. For corporations, it highlights the need for robust governance frameworks that align with both regulatory expectations and investor protection standards.
As the lead plaintiff deadline of September 22, 2025, approaches[1], the case will likely influence how ophthalmic tech firms approach transparency. In an industry where innovation and compliance are inextricably linked, the RxSight saga reinforces the adage: in securities law, silence is not always golden.

Comentarios
Aún no hay comentarios