IDEAYA Biosciences' Darovasertib: A Breakthrough in Uveal Melanoma Therapy?
In the rare and aggressive cancer landscape of (UM), IDEAYA BiosciencesIDYA-- has emerged as a disruptive force with its (PKC) inhibitor, . Recent Phase 2 trial data and regulatory advancements position the drug as a potential game-changer in both neoadjuvant and metastatic settings, addressing a market with significant unmet needs and, according to an Mordor Intelligence market report, a projected value of . This analysis evaluates darovasertib's clinical promise and commercial potential, contextualized within the evolving uveal melanoma treatment ecosystem.
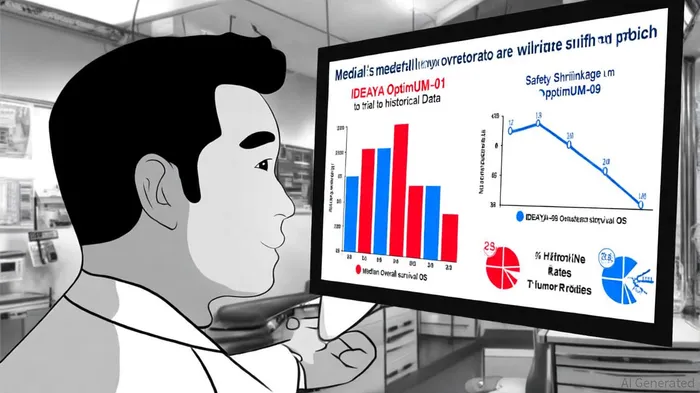
Clinical Efficacy: A Dual-Pronged Approach
IDEAYA's trial has yielded compelling results in first-line metastatic uveal melanoma (mUM). The combination of darovasertib and crizotinib achieved a , , according to an IDEAYA news release. , coupled with a and , underscores the therapy's potential to redefine treatment standards for mUM. The same release also reported manageable adverse events (e.g., diarrhea, fatigue) predominantly at Grades 1–2.
In the neoadjuvant setting, has demonstrated darovasertib's ability to shrink primary tumors, with and , according to an IDEAYA interim data release. That announcement also noted , . For patients initially recommended for enucleation (eye removal), the drug's efficacy translated into a , rising to , as reported in a Financial Times announcement. These outcomes highlight darovasertib's capacity to delay or avoid irreversible interventions, a critical value proposition in primary UM management.
Regulatory Momentum and Market Positioning
The U.S. FDA's (BTD) for darovasertib in neoadjuvant UM marks a pivotal regulatory milestone, according to an IDEAYA FDA announcement. This designation, granted based on OptimUM-09's interim data, expedites development pathways and signals regulatory confidence in the drug's transformative potential. IDEAYAIDYA-- is now advancing OptimUM-02, a Phase 2/3 trial evaluating the darovasertib-crizotinib combination in HLA*A2:01-negative mUM, with median progression-free survival (PFS) data expected by early 2026 to support accelerated approval, as previously reported in the company's news release.
Competitively, darovasertib's dual utility in neoadjuvant and metastatic settings differentiates it from existing therapies. While tebentafusp (Immunocore) and (Iovance Biotherapeutics) dominate the mUM space with survival benefits, , where systemic therapies remain unapproved, according to the Mordor report. With OptimUM-10, a global Phase 3 neoadjuvant trial initiated in Q3 2025, IDEAYA is poised to capture a significant share of this underserved population, as noted in the company's FDA announcement.
Commercial Viability: Navigating a High-Stakes Market
The uveal melanoma treatment market is projected to grow at a , , per the Mordor Intelligence analysis. IDEAYA's focus on -a novel mechanism in UM-positions darovasertib to outperform traditional radiation and enucleation, which carry high morbidity risks. Additionally, the drug's favorable safety profile and potential for combination therapy (e.g., with crizotinib) enhance its commercial allure.
However, challenges persist. The market is increasingly competitive, with players like Bristol-Myers Squibb and AstraZeneca advancing immunotherapies and gene therapies, as highlighted by industry analysts. Moreover, the high cost of personalized treatments and limited patient pools for trials could strain scalability. Yet, IDEAYA's BTD and robust Phase 2 data provide a strong foundation for navigating these hurdles, particularly as the company aligns with payers and regulators to demonstrate cost-effectiveness.
Conclusion: A Transformative Investment Opportunity
IDEAYA Biosciences' darovasertib represents a paradigm shift in uveal melanoma care, offering unprecedented efficacy in both early and advanced disease. With Breakthrough Therapy Designation, a , and a tailwind, the drug's clinical and commercial potential is formidable. As OptimUM-02 and OptimUM-10 progress, investors should closely monitor and regulatory updates, which could catalyze a revaluation of IDEAYA's pipeline. In a market starved for innovation, darovasertib's dual therapeutic versatility and regulatory momentum make it a compelling candidate for long-term investment.

Comentarios
Aún no hay comentarios