Humacyte's Potential as a Biotech Breakout: Market Validation and Clinical Progress Post-Symvess Humanitarian Trial
Humacyte's Symvess has emerged as a transformative solution in vascular trauma care, with recent clinical and commercial milestones validating its potential as a biotech breakout. The product's performance in real-world combat settings and hospital environments, coupled with regulatory approvals and early market traction, positions the company for significant growth.
Clinical Validation: A New Standard in Vascular Trauma Care
According to a GlobeNewswire release reporting an Oxford Academic Military Medicine paper, Symvess demonstrated exceptional outcomes in treating wartime vascular trauma in Ukraine. A 18-month follow-up of 17 patients showed 100% limb salvage, zero infections or amputations, and an 87.1% patency rate (GlobeNewswire release). These results align with earlier 30-day data, which reported a 93.8% patency rate. Dr. Oleksandr Sokolov, a Ukrainian vascular surgeon, emphasized that Symvess reduces surgical reconstruction time and acute ischemia duration, critical advantages in battlefield scenarios.
Further validation comes from a Journal of Vascular Surgery study, where Symvess achieved 92% secondary patency in 12 patients with hospital-acquired vascular complications, with 100% limb salvage and no infections, as detailed in a QuiverQuant report (QuiverQuant report). These findings underscore Symvess's versatility, offering a practical alternative to autologous vein grafts, which require time-consuming harvesting and are often infeasible in urgent settings.
Regulatory and Commercial Momentum
Humacyte's December 2024 FDA approval marked a pivotal transition from development to commercialization. By July 2025, the company secured ECAT approval, enabling Symvess distribution to 35 U.S. Military Treatment Facilities and 160 VA hospitals. This expansion was swiftly followed by the first commercial sale to a major military medical complex in July 2025, signaling robust demand in high-acuity settings.
Early market adoption is also evident in civilian healthcare. As of March 2025, 34 hospitals had initiated Value Analysis Committee (VAC) approval processes, with three completing them. This progress reflects growing recognition of Symvess's clinical value, particularly in complex vascular trauma cases where traditional grafts fall short.
Financials and Future Prospects
While Humacyte's Q2 2025 revenue of $301,000 and H1 2025 total of $818,000 remain modest, the company's $38.0 million in cash reserves as of June 30, 2025, provide a buffer for scaling operations (StockTitan report). The key to long-term success lies in expanding Symvess's indications. In 2025, HumacyteHUMA-- plans to file an Investigational New Drug (IND) application for coronary artery bypass grafting studies, a move that could unlock a multibillion-dollar market. A supplemental Biologics License Application (BLA) is also slated for late 2026, further broadening Symvess's therapeutic applications.
Conclusion: A Biotech Breakout in the Making
Humacyte's Symvess has achieved a rare trifecta: clinical excellence, regulatory clearance, and early commercial traction. The product's performance in high-stakes environments-from war zones to hospital operating rooms-demonstrates its ability to address unmet medical needs. With a clear path to expand into coronary applications and a growing footprint in military and veteran healthcare, Humacyte is well-positioned to transition from a niche biotech to a market leader. For investors, the combination of de-risked clinical data and scalable commercial potential makes Symvess a compelling bet on the future of regenerative medicine.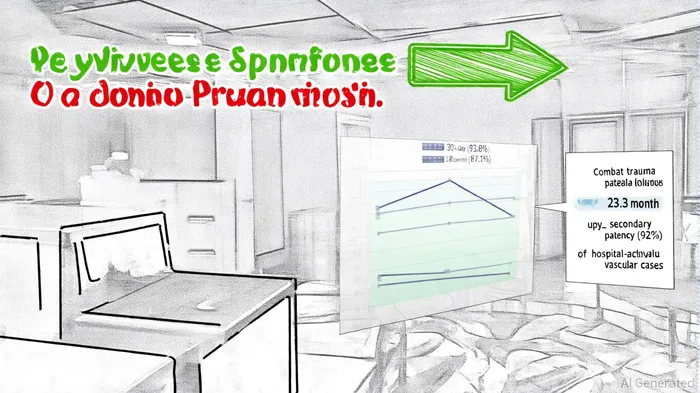

Comentarios
Aún no hay comentarios