Heartflow's FDA 510(k) Clearance and its Implications for Cardiovascular Diagnostics
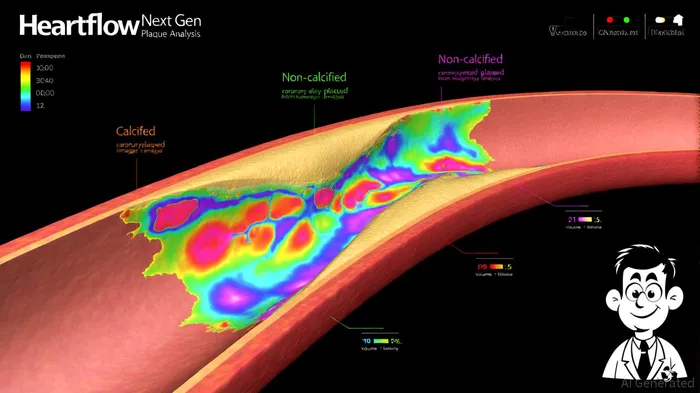
The recent FDA 510(k) clearance for Heartflow's Next Gen Plaque Analysis algorithm marks a pivotal moment in the evolution of AI-powered cardiovascular diagnostics. According to a report by GlobeNewswire, the updated platform—cleared on September 22, 2025—demonstrates a 21% improvement in plaque detection compared to its predecessor, leveraging a dataset of approximately 273,000 patients—nine times larger than any existing plaque quantification study[1]. This leap in accuracy, coupled with advanced 3D visualization tools for plaque type, volume, and distribution, positions HeartflowHTFL-- to redefine clinical workflows for coronary artery disease (CAD) management[1].
Accelerated Market Adoption: Coverage and Clinical Validation
Heartflow's strategic alignment with payers is equally transformative. Cigna's nationwide coverage of the Plaque Analysis platform, effective October 1, 2025, follows a similar decision by UnitedHealthcare, signaling broad industry validation[1]. This coverage extends to patients with mild-to-moderate stenosis (1-69%) identified via coronary CTA, a population often underserved by traditional diagnostics. Data from Heartflow's DECIDE Registry further strengthens the case for adoption: the platform prompted medical management changes in over 50% of patients and is projected to reduce cardiac events by 15%[1]. Such outcomes not only justify cost-effectiveness but also align with updated clinical guidelines prioritizing non-invasive diagnostics.
Competitive Differentiation in a Crowded AI Landscape
Heartflow's competitors in AI-driven cardiac imaging—such as Cleerly, Artrya, and Caristo Diagnostics—offer innovative solutions but lack the regulatory and reimbursement tailwinds propelling Heartflow's growth[2]. While these firms focus on niche applications like coronary CTA analysis or digital twins, Heartflow's FDA clearance and payer partnerships create a moat. The company's algorithm achieves 95% agreement with intravascular ultrasound (IVUS), the gold standard for plaque assessment, while avoiding the invasiveness and costs of procedures like angiography[1]. Moreover, the scale of its training data—nine times larger than peer studies—ensures robust generalizability, a critical factor in clinician adoption.
Investment Implications
For investors, Heartflow's clearance represents more than a regulatory win—it signals a structural shift in cardiovascular care. The integration of AI with payer coverage and clinical evidence creates a flywheel effect: improved outcomes drive broader adoption, which in turn attracts payers and providers. Competitors without similar regulatory or reimbursement milestones may struggle to catch up, particularly as AI models require vast datasets to achieve comparable accuracy. As the U.S. grapples with rising CAD prevalence, Heartflow's platform is poised to become a standard of care, accelerating the transition from reactive to preventive cardiology.

Comentarios
Aún no hay comentarios