HANSIZHUANG's Gastric Cancer Breakthrough and the Path to Accelerated Market Access
The landscape of gastric cancer treatment is undergoing a seismic shift, driven by immunotherapies that promise to redefine survival outcomes and quality of life. At the forefront of this transformation is HANSIZHUANG (serplulimab), a PD-1 inhibitor developed by Henlius. Recent phase 3 trial data from the ASTRUM-006 study has positioned HANSIZHUANG as a groundbreaking candidate in the neoadjuvant/adjuvant treatment of early-stage gastric cancer, with implications that extend far beyond clinical efficacy into regulatory strategy and market access.
A Paradigm Shift in Gastric Cancer Care
According to a PR Newswire report, the ASTRUM-006 trial demonstrated that HANSIZHUANG combined with chemotherapy achieved a statistically significant improvement in event-free survival (EFS) and a more than threefold increase in pathological complete response (pCR) rates compared to chemotherapy alone (a PR Newswire report). This is the first regimen to replace adjuvant chemotherapy with immunotherapy in the perioperative setting, offering a less toxic alternative with a favorable safety profile. The mechanism of action-enhanced PD-1 internalization and sustained T-cell activation-further underscores its potential across solid tumors.
These results align with a broader trend in oncology toward personalized medicine. As noted in a 2023 PubMed review, biomarkers such as PD-L1, microsatellite instability (MSI), and HER2 are increasingly guiding treatment decisions, enabling therapies like HANSIZHUANG to target specific patient subpopulations more effectively (a 2023 PubMed review). For gastric cancer, where treatment options remain limited, this precision could translate into a significant unmet medical need being addressed.
Regulatory Pathways: Fast Track or Breakthrough?
Despite the clinical promise, HANSIZHUANG has not yet received FDA Breakthrough Therapy Designation for gastric cancer, as of September 2025. However, its regulatory trajectory is not without momentum. In Europe, the Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion for HANSIZHUANG as a first-line treatment for extensive-stage small cell lung cancer (ES-SCLC), marking it as a potential first-in-class anti-PD-1 monoclonal antibody in that indication. In China, it is already approved for multiple indications, including microsatellite instability-high (MSI-H) solid tumors and esophageal squamous cell carcinoma (ESCC), according to Henlius' announcement (Henlius' announcement).
The absence of a Breakthrough Therapy Designation for gastric cancer does not preclude accelerated pathways. HANSIZHUANG's Orphan Drug Designation for small cell lung cancer in the U.S. provides a framework for leveraging incentives such as tax credits and expedited reviews, as reported in a BioSpace article (a BioSpace article). For gastric cancer, the phase 3 trial's robust data may qualify it for Fast Track Designation, which offers more frequent FDA interactions and potential accelerated approval. Notably, a competing therapy, SIGX1094, was granted Fast Track for diffuse gastric cancer in February 2025, signaling the FDA's openness to innovative therapies in this space.
Market Access and Strategic Implications
HANSIZHUANG's commercial potential hinges on its ability to navigate regulatory hurdles while capitalizing on existing approvals. In China, where it is already marketed for multiple cancers, the drug benefits from a first-mover advantage and a well-established distribution network. In Europe, the CHMP's positive opinion for ES-SCLC opens doors to broader oncology adoption, particularly as payers increasingly prioritize cost-effective, high-impact therapies.
For the U.S. market, the absence of Breakthrough Therapy Designation for gastric cancer may necessitate a dual strategy: leveraging Orphan Drug benefits for SCLC while pursuing Fast Track status for gastric cancer based on ASTRUM-006's results. This approach mirrors the path of other immunotherapies that secured approvals through niche indications before expanding into larger markets.
Conclusion: A Calculated Leap Forward
HANSIZHUANG's breakthrough in gastric cancer is not merely a clinical achievement but a strategic inflection point. While the lack of an FDA Breakthrough Therapy Designation for this indication may slow U.S. entry, the drug's phase 3 results and existing approvals in China and Europe position it as a formidable contender in the global oncology market. Investors should monitor Henlius's NDA strategy closely, particularly its ability to leverage Fast Track Designation and biomarker-driven patient selection to accelerate market access. In an era where speed and precision define therapeutic success, HANSIZHUANG's journey offers a compelling case study in navigating the intersection of innovation and regulation.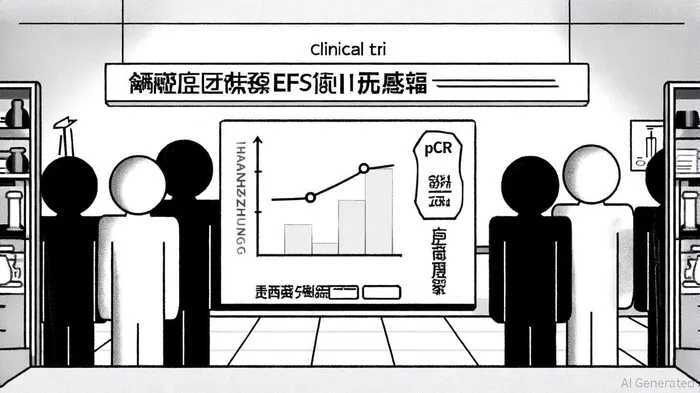



Comentarios
Aún no hay comentarios