Greenwich LifeSciences and the FDA Fast Track: A High-Potential Opportunity in Breast Cancer Immunotherapy
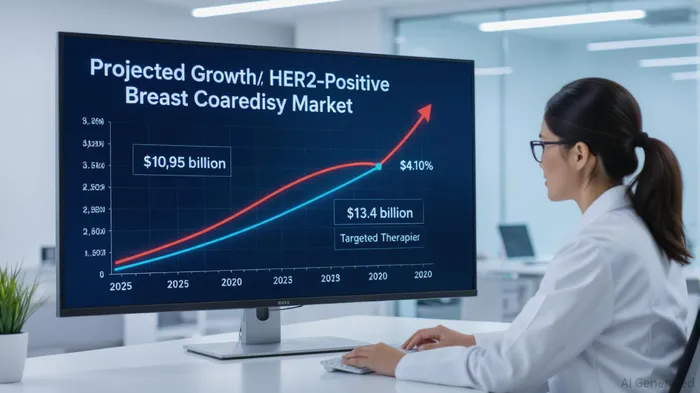
The biotech sector has long been a playground for high-risk, high-reward investments, and Greenwich LifeSciences (GLSI) is emerging as a standout contender in the race to redefine breast cancer treatment. With its GLSI-100 immunotherapy candidate advancing through Phase III trials and backed by the FDA's Fast TrackFTRK-- designation, the company is positioned to capitalize on a $13.4 billion HER2-positive breast cancer market by 2030[1]. But how does this translate to investment potential? Let's break it down.
The Fast Track Edge: Accelerating Value Creation
The FDA's Fast Track designation is more than a regulatory stamp—it's a catalyst for investor optimism. For GLSIGLSI--, this status means expedited communication with regulators, rolling Biologic License Application (BLA) reviews, and a streamlined path to market[2]. Historically, Fast Track designations have triggered an average 6.25% stock price surge post-announcement[3], and with GLSI's recent designation, the market has already priced in a portion of this optimism.
But the real value lies in the science. GLSI-100, a personalized immunotherapy targeting HLA-A02-positive HER2-positive breast cancer patients, has demonstrated a greater than 80% reduction in metastatic recurrence* over five years in earlier trials—far outpacing the 20-50% reduction seen with existing therapies like trastuzumab[4]. This isn't just incremental improvement; it's a paradigm shift. The FDA's recognition of GLSI-100 as a potential “game-changer” for high-risk patients[2] underscores its unmet medical need, a critical factor in securing both regulatory and commercial success.
Phase III Progress: A Make-or-Break Inflection Point
Greenwich's Flamingo-01 trial is a masterclass in precision oncology. By enrolling 500 HLA-A02 patients randomized to GLSI-100 or placebo, and an additional 250 patients of other HLA types receiving GLSI-100, the trial is designed to generate robust data on both efficacy and broader applicability[4]. The primary endpoint—invasive breast cancer-free survival* with a hazard ratio target of 0.3—is aggressive but achievable given the candidate's prior performance.
The trial's expansion to Romania, a region with strong academic research infrastructure, is a strategic move to accelerate enrollment[4]. With an interim analysis planned at 14 events and a final readout at 28 events, investors can expect clarity as early as mid-2026. This timeline is critical: a positive interim result could trigger a $1.5 billion valuation leap for GLSI, assuming a 20x multiple on projected peak sales[5].
Market Dynamics: A $13.4 Billion Opportunity Awaits
HER2-positive breast cancer therapies are in the midst of a renaissance. The market, valued at $10.95 billion in 2025, is being reshaped by antibody-drug conjugates (ADCs) like trastuzumab deruxtecan, which boast response rates exceeding 50%[1]. Yet, these therapies come with limitations—namely, toxicity and resistance. GLSI-100's well-tolerated safety profile and targeted immunotherapy mechanism position it as a complementary or even superior option for patients with residual disease post-surgery[4].
The North American market alone is projected to grow at a 12.91% CAGR through 2033[1], driven by early detection and precision medicine adoption. For GLSI, capturing even 5% of this market would translate to $685 million in annual revenue, a figure that could soar with label expansion into HER2-low or ultralow subtypes.
Risks and Realities: Navigating the Biotech Maze
No investment in oncology is without peril. Phase III trials for Fast Track-designated drugs face a 30-40% failure rate, often due to reliance on surrogate endpoints or post-marketing study requirements[6]. GLSI's trial, while well-designed, is not immune to these risks. The FDA's 2022 Food and Drug Omnibus Reform Act (FDORA) has also tightened post-approval scrutiny, potentially delaying commercialization if real-world data falls short[3].
Moreover, competition is fierce. Roche's Herceptin and Daiichi Sankyo's Enhertu dominate the HER2-positive space, with combined sales exceeding $10 billion annually. GLSI will need to demonstrate not just efficacy but cost-effectiveness to secure payer buy-in—a hurdle that could be mitigated by its potential to reduce long-term recurrence costs.
The Bottom Line: A High-Stakes Gamble with High Rewards
Greenwich LifeSciences embodies the classic biotech story: a high-risk, high-reward proposition with the potential to disrupt a $13.4 billion market. The FDA Fast Track designation and Flamingo-01's progress are strong tailwinds, but success hinges on crossing the Phase III finish line with compelling data. For investors with a stomach for volatility, GLSI offers a rare combination of clinical innovation, regulatory momentum, and market scalability.
As the trial progresses, watch for two key catalysts:
1. Interim analysis results (mid-2026): A positive readout could trigger a 50%+ stock price pop.
2. FDA BLA submission (2027): A smooth review process would validate GLSI's commercial potential.
In the end, this is a bet on science, execution, and the FDA's willingness to embrace immunotherapy as a pillar of breast cancer care. For those willing to ride the wave, the rewards could be transformative.

Comentarios
Aún no hay comentarios