GH Research's IND Update and Phase 3 Program: A Catalyst for Long-Term Value Creation in Treatment-Resistant Depression
The biopharmaceutical sector thrives on catalyst-driven momentum, and GH Research PLCGHRS-- (GHRS) has positioned itself as a compelling case study in this paradigm. With its lead candidate, GH001-a proprietary inhalation formulation of mebufotenin-advancing toward pivotal Phase 3 trials for treatment-resistant depression (TRD), the company is poised to deliver a series of high-impact events that could redefine its valuation trajectory. The upcoming IND update on January 5, 2026, and the initiation of its global Phase 3 program in 2026 represent not just regulatory milestones but also strategic inflection points for investors seeking exposure to transformative therapies in a rapidly expanding market.
Phase 2b Success: A Foundation for Pivotal Trials
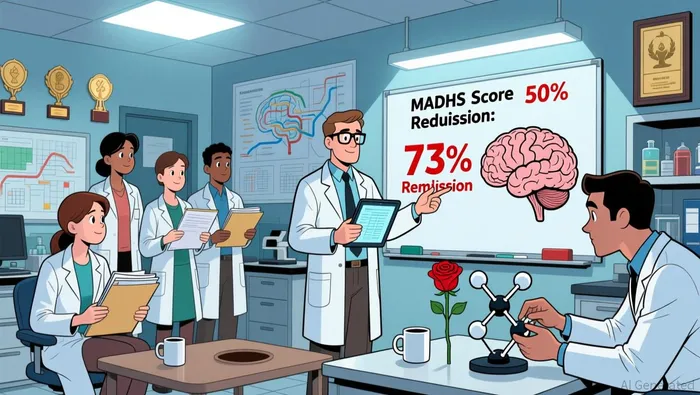
GH001's Phase 2b trial (GH001-TRD-201) has already demonstrated robust clinical proof of concept. The trial met its primary endpoint with a statistically significant placebo-adjusted reduction of -15.5 points in Montgomery-Åsberg Depression Rating Scale (MADRS) scores on Day 8 (p<0.0001) according to clinical data. More impressively, the open-label extension (OLE) confirmed a 73% remission rate at six months, achieved with infrequent treatment visits and no mandated psychotherapy according to the company. These results, coupled with a safety profile marked by no treatment-related serious adverse events over six months as reported, underscore GH001's potential to disrupt the TRD treatment landscape.
Regulatory Progress: Clearing the Final Hurdle
GH Research has navigated a critical regulatory bottleneck, addressing all but one of the FDA's clinical hold topics related to its IND application for GH001. The remaining issue-respiratory tract histology findings in rats-has been deemed rat-specific by the company, with no extrapolation to human risk according to GH Research. This resolution, expected to be finalized by early 2026, will pave the way for the Phase 3 program. The company's proactive engagement with the FDA, including preparations for an end-of-Phase 2 meeting, further signals its readiness to scale the program efficiently according to QuiverQuant.
Market Opportunity: A $3.14 Billion Target by 2030
The TRD market is a high-growth segment, projected to expand from $2.16 billion in 2025 to $3.14 billion by 2030, reflecting a 7.75% compound annual growth rate (CAGR). GH001's mechanism-delivering mebufotenin via a proprietary inhalation method-offers a distinct advantage over existing therapies. Unlike esketamine (Spravato) or psilocybin-based candidates, GH001's rapid onset of action and minimal need for psychotherapeutic support align with patient-centric care models. Its ability to achieve remission with infrequent visits also reduces healthcare system burden, a critical differentiator in value-based care environments as confirmed by GH Research.
Phase 3 Design: Building on Promising Data
While specific details on the Phase 3 trial design remain undisclosed, the company has indicated that the program will build on the Phase 2b results. Key endpoints are expected to include MADRS remission rates (≤10) and long-term durability of response, with adaptive elements such as individualized dosing regimens to optimize outcomes according to company information. The Phase 2b trial's 16-patient cohort, though small, provided a strong signal of efficacy, suggesting that the Phase 3 program could be streamlined to focus on confirming these results in a larger population.
Strategic Catalysts and Investment Implications
For catalyst-driven investors, GH Research's roadmap is laden with high-impact events. The January 5, 2026, IND update will provide clarity on regulatory readiness, while the initiation of Phase 3 trials in 2026 will serve as a proxy for commercial momentum. If successful, these milestones could unlock significant value, particularly given the TRD market's projected growth and GH001's favorable risk-benefit profile. Additionally, the company's parallel development of GH002, an intravenous mebufotenin formulation, adds a secondary catalyst with an anticipated IND submission in Q4 2025 according to GH Research.
Conclusion: A High-Conviction Play in a Transformative Space
GH Research's journey from Phase 2b success to Phase 3 readiness exemplifies the power of clinical-stage biopharma investing. With a clear regulatory path, a differentiated therapy, and a growing market, the company is well-positioned to deliver outsized returns for investors who recognize the inflection points ahead. As the January 2026 update approaches, the focus will shift from potential to execution-a transition that often defines the difference between speculative plays and sustainable value creation.

Comentarios
Aún no hay comentarios