GC Biopharma's Strategic Move: Acquiring Shingles Vaccine CMO Rights to Cement Leadership in a High-Growth Sector
GC Biopharma's recent acquisition of contract manufacturing organization (CMO) rights for its shingles vaccine candidate, amezosvatein, from its U.S. affiliate Curevo marks a pivotal strategic move in the high-growth vaccine manufacturing sector. This transaction, coupled with Curevo's $110 million Series B funding round, according to a GlobeNewswire release, underscores GC Biopharma's ambition to challenge GSK's Shingrix dominance while leveraging its internal mRNA platform and global manufacturing capabilities.
Market Opportunity and Strategic Rationale
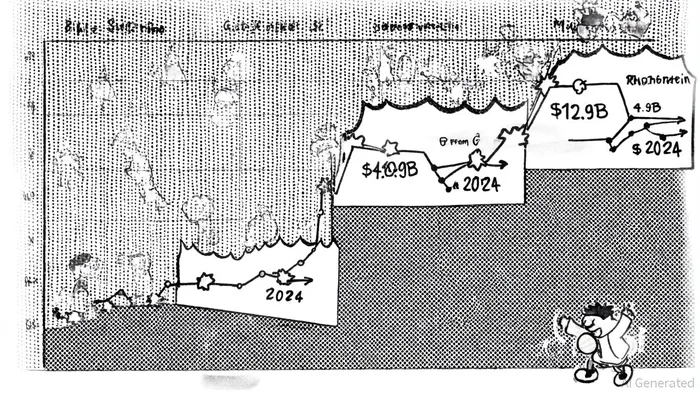
The global shingles vaccine market, valued at $4.9 billion in 2024, is projected to reach $12.9 billion by 2034, driven by aging populations and expanding immunization programs, according to a GM Insights report. Shingrix, GSK's recombinant adjuvanted vaccine, currently dominates the market but faces competition from emerging technologies, including mRNA-based candidates from Pfizer/BioNTech and SK Bioscience's SKYZoster, as reported in a Korea Biomed report. GC Biopharma's Amezosvatein, a non-mRNA subunit vaccine targeting glycoprotein E (gE), aims to differentiate itself through improved tolerability while maintaining high efficacy. The vaccine's adjuvant, an optimized TLR4 agonist developed by Seattle's Access to Advanced Health Institute (AAHI) and licensed from GC Biopharma-funded Mogam Institute, is designed to reduce side effects common in existing vaccines, according to a Wowtale article.
Curevo's CEO, George Simeon, emphasized that the $110 million funding will accelerate Phase 2 trials, including 640 participants aged 70 and older, to finalize dosing for Phase 3 trials by mid-2025, per a Korea Biomed report. GC Biopharma's role as a co-investor and its ownership of the Mogam Institute highlight its deep commitment to the project, aligning with its broader strategy to address unmet needs in geriatric vaccine development, as detailed in a Business Korea article.
Strengthening Manufacturing and R&D Capabilities
GC Biopharma's strategic positioning is further bolstered by its end-to-end mRNA vaccine platform, developed internally since 2023. The company's Hwasun, South Jeolla-based facility enables full control over mRNA synthesis, LNP formulation, and quality control, making it the first Korean firm to internalize the entire mRNA drug production process, according to a Pharmaceutical-Technology article. This capability not only supports its own pipeline, including a COVID-19 mRNA vaccine (GC4006A), but also positions it to scale Amezosvatein production efficiently.
The acquisition of CMO rights for amezosvatein complements GC Biopharma's existing vaccine portfolio, which includes Varicella and influenza vaccines contributing to overseas sales growth, as reported in a Pulse report. By integrating Curevo's vaccine candidate into its manufacturing network, GC Biopharma can reduce reliance on third-party CMOs and accelerate time-to-market-a critical advantage in a sector where regulatory and supply chain delays are common.
Competitive Landscape and Risk Mitigation
While GSK's Shingrix remains the market leader, Curevo's adjuvant technology, validated by former GSKGSK-- executive Moncef Slaoui, offers a compelling value proposition, according to Korea Biomed. Slaoui's involvement, alongside Curevo's board member Dr. Tal Zaks (formerly of Moderna), signals strong scientific credibility. Additionally, Curevo's focus on elderly populations-a demographic with high shingles incidence-aligns with global public health priorities, including WHO's anticipated updates on herpes zoster vaccine recommendations, as noted in a Mordor Intelligence report.
However, challenges persist. Clinical trial delays or suboptimal Phase 3 results could hinder Amezosvatein's commercialization. Moreover, mRNA-based competitors like Pfizer/BioNTech may leverage their platform advantages to enter the market. GC Biopharma's response to these risks lies in its diversified R&D strategy, which includes applying its mRNA platform to rare diseases and cancer therapeutics, thereby spreading innovation risks across multiple therapeutic areas, according to a PR Newswire release.
Conclusion
GC Biopharma's acquisition of CMO rights for Amezosvatein represents a calculated move to secure a leadership position in the $12.9 billion shingles vaccine market. By combining its mRNA manufacturing expertise, strategic funding of Curevo, and focus on tolerability-driven innovation, the company is well-positioned to challenge incumbents while capitalizing on demographic and regulatory tailwinds. As the global demand for vaccines intensifies, GC Biopharma's integrated approach to R&D and production could serve as a blueprint for next-generation vaccine manufacturers.

Comentarios
Aún no hay comentarios