Eli Lilly Q2 Earnings: Weight loss drugs build patient dependence; expanded production boosts advantage
Recently, the U.S. stock market's weight loss drug duo, Eli Lilly and Novo Nordisk, have both released their financial reports. As one of the most highly anticipated sectors beyond AI, the performance of these two companies is closely watched. After Novo Nordisk's setback, Eli Lilly made a strong comeback, demonstrating significant potential in the weight loss drug industry!
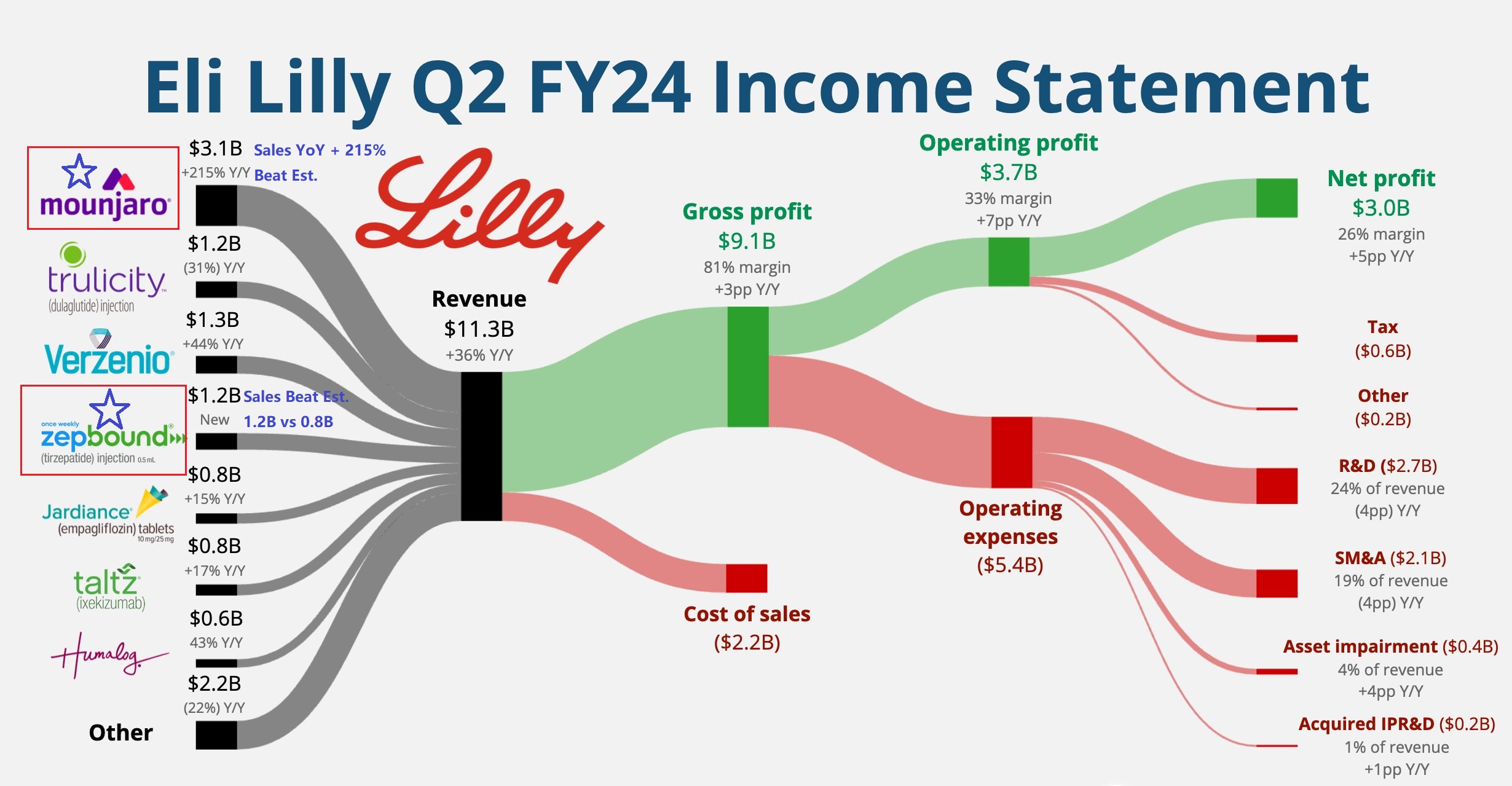
Specifically, Eli Lilly reported Q2 revenue of $11.3 billion, surpassing expectations of $9.98 billion and marking a 36% year-over-year increase; earnings per share surged by 86% year-over-year.
The full-year revenue guidance has been raised to $45.4 billion – $46.6 billion (previously $42.4 billion – $43.6 billion); adjusted earnings per share have been increased to $16.10 – $16.60 (previously projected at $13.70).
Growth in the second half of the year is expected to accelerate, primarily driven by the strong performance of the miracle weight loss drugs Mounjaro and Zepbound. Due to robust demand, weight loss drugs are anticipated to see price increases; other drugs will maintain the same pricing as in the first half of the year, with no plans for promotional activities. Additionally, the company plans to launch more new products and expand the sales of existing ones in the latter half of the year.
Eli Lilly's earnings call highlights:
- Increased investment to expand the production capacity of Zepbound and Mounjaro. Since 2020, over $18 billion has been invested in expanding manufacturing in the U.S. and Europe, yielding positive returns. Following Novo Nordisk's acquisition of Catalent (a major supplier to Eli Lilly) in February of this year, Eli Lilly is focusing on building its own manufacturing facilities.
- Plans to introduce 2.5 mg and 5 mg single-dose bottles of Zepbound in the coming weeks to further expand its patient base. As of July 1, over 50% of U.S. employers are providing medical insurance for anti-obesity drugs, a proportion that is continuing to grow and benefiting more patients.
- Patients using Eli Lilly's weight loss drugs have experienced benefits, such as weight loss and treatment for other conditions, leading to dependency and encouraging patients to complete the full treatment course.
In addition to its weight loss drugs, Eli Lilly has made strides in other pharmaceuticals:
- In July, the FDA approved Eli Lilly's Kisunla for treating early-stage Alzheimer's disease (AD) in adults. Some patients have already begun using this drug for clinical treatment.
- Eli Lilly's tirzepatide has met all primary and key secondary endpoints in Phase 3 trials for treating moderate to severe obstructive sleep apnea and obesity; it also shows potential for treating heart failure. The FDA is expected to review it by the end of 2024. There is significant interest in studying tirzepatide's benefits in reducing absenteeism and improving productivity in the workplace.
- Currently, there are 11 new obesity drugs in clinical trials, covering various indications; Eli Lilly has also made significant progress in oncology and neurology.

What competitors are weight loss drugs facing, and how is Eli Lilly addressing competition?
Previously, up-and-coming weight loss drug developer Viking Therapeutics accelerated the development of its candidate VK2735. After receiving written feedback from the FDA, the company decided to advance it to Phase 3 (final) clinical trials.
Swiss pharmaceutical giant Roche announced that its subsidiary Carmot Therapeutics' second weight loss drug candidate showed positive effects in early trials, with Roche's first weight loss drug potentially hitting the market earlier than the expected 2028.
In response to market competition, Eli Lilly's management stated during the call:
- The weight loss drug market is large, and competitors will inevitably come, but the duo (Eli Lilly and Novo Nordisk) already has a significant first-mover advantage. To catch up, competitors face technical difficulties, high capital investment requirements, and the time needed for large-scale production. We are already far ahead.

What risks are faced?
Analysts in the call asked how Eli Lilly addresses risks of infringement and being infringed upon, as well as the risk of drug contamination due to lax oversight by licensed manufacturers, potentially damaging the company's reputation. Eli Lilly executives acknowledged these risks and expressed significant concern but did not provide specific solutions, only stating they would strengthen cooperation with regulatory agencies to mitigate these risks.

Why did Novo Nordisk's profits fall short of expectations despite being a weight loss drug leader?
Novo Nordisk's profits fell short of expectations partly due to asset impairment. A late-stage kidney disease trial failed in June, resulting in a 5.7 billion Danish krone (approximately $835 million) impairment loss. The pharmaceutical R&D field is inherently risky, and failures can lead to substantial losses, which is a risk inherent in investing in the pharmaceutical industry.

Novo Nordisk's management mentioned during the call that the company's Wegovy weight loss drug saw a price reduction, but this still met executive expectations. The price drop was due to promotional pricing to capture market share. The company also withdrew its submission to regulatory authorities for Wegovy's approval for treating heart failure, with plans to resubmit early next year. Additionally, increased costs from factory expansions have also pressured profit margins.
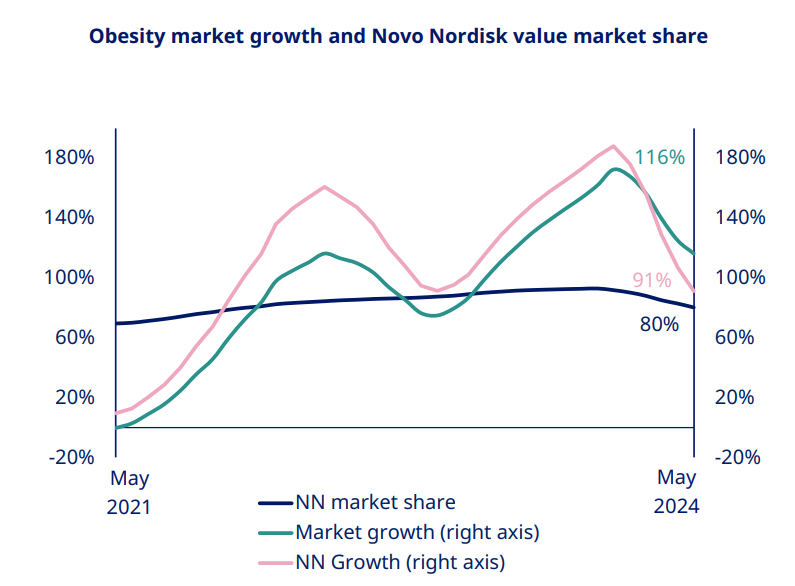
The company's guidance indicates that Novo Nordisk's market share has peaked and is declining, with revenue growth trailing the overall market growth of weight loss drugs. This has also contributed to pressure on its stock price.

Comentarios
Aún no hay comentarios