Eisai's Leqembi IQLIK: A Paradigm Shift in Alzheimer's Treatment and Market Access
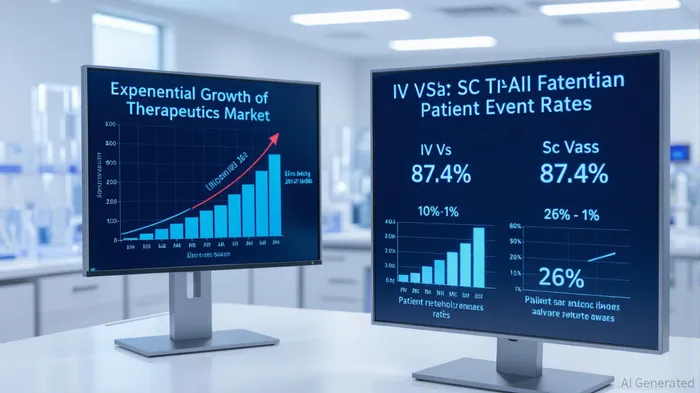
The Alzheimer’s disease therapeutics market is on the cusp of a transformative era, driven by breakthroughs in disease-modifying therapies (DMTs) and innovations in patient-centric delivery. At the forefront of this shift is Eisai’s Leqembi IQLIK, a subcutaneous autoinjector formulation of lecanemab, which has redefined the treatment landscape by enabling at-home administration. With the global Alzheimer’s therapeutics market projected to grow at a staggering 23.4% CAGR—surpassing $19.3 billion by 2033 [2]—Eisai’s strategic pivot to subcutaneous dosing positions it to capture a significant share of this expanding pie.
A Clinical and Commercial Game-Changer
Leqembi IQLIK’s approval by the FDA in January 2025 marked a pivotal moment. The subcutaneous autoinjector, which requires just 15 seconds of administration time, eliminates the logistical and physical burdens of intravenous (IV) infusions [4]. Clinical trials demonstrate that the subcutaneous formulation maintains equivalent efficacy to IV dosing while reducing systemic adverse events to less than 1% (versus 26% for IV) and achieving a remarkable 87.4% patient retention rate over two years [1]. This is a stark contrast to the 40% retention rate observed with IV therapy, underscoring the formulation’s potential to sustain long-term adherence—a critical factor in chronic disease management.
Eisai’s aggressive market strategy is already bearing fruit. By Q2 2025, lecanemab held a 70% market share in the Alzheimer’s drug sector, with global sales reaching $160 million [2]. The subcutaneous version is projected to capture 60% of the lecanemab market by 2026 [1], driven by its convenience and safety profile. Eisai’s pricing model—$19,500 annually before rebates—balances accessibility with profitability, ensuring broad patient access while maintaining revenue upside [4].
Market Expansion and Revenue Potential
The subcutaneous formulation’s impact extends beyond clinical outcomes. By enabling home administration, Leqembi IQLIK addresses a critical barrier to treatment: geographic and infrastructural limitations. Rural areas, where access to infusion centers is sparse, now represent untapped markets. Eisai’s partnerships and patient support programs further enhance accessibility, aligning with global efforts to decentralize healthcare delivery [4].
Financially, the drug is on a trajectory to become a blockbuster. Eisai projects Leqembi IQLIK to generate $1.6–$1.8 billion in sales by 2027 [1], with U.S. revenue increasing by 20% in Q3 2025 alone to $157 million [4]. These figures are bolstered by the broader market’s growth dynamics: the DMT segment alone is expected to expand at a 67.8% CAGR, reaching $13.1 billion by 2030 [1]. Eisai’s first-mover advantage in subcutaneous DMTs, combined with its 70% market share in Alzheimer’s drugs, positions it to dominate this high-growth niche.
Strategic Risks and Opportunities
While the outlook is optimistic, challenges remain. The subcutaneous formulation currently requires an 18-month IV initiation phase, limiting its immediate standalone use. However, Eisai and BiogenBIIB-- are pursuing regulatory approval for a subcutaneous-only regimen, which could unlock broader adoption [3]. Additionally, pricing pressures from payers and competitors may emerge as the market matures. Yet, Eisai’s robust clinical data and patient retention metrics provide a strong defense against such headwinds.
The long-term revenue potential is further amplified by the pipeline of combination therapies. With the FDA approval of Leqembi IQLIK paving the way for multi-drug regimens [3], Eisai could leverage its market leadership to integrate complementary treatments, enhancing both patient outcomes and revenue streams.
Conclusion
Eisai’s Leqembi IQLIK is not merely a product—it is a paradigm shift in Alzheimer’s care. By merging clinical innovation with market accessibility, the subcutaneous autoinjector addresses unmet needs across the care continuum. As the Alzheimer’s therapeutics market surges toward $19.3 billion by 2033 [2], Eisai’s strategic positioning—backed by a 70% market share and a 67.8% CAGR in DMTs [1]—makes Leqembi IQLIK a compelling investment. For investors, the question is not whether this market will grow, but how quickly Eisai can consolidate its dominance in the new era of at-home Alzheimer’s treatment.
**Source:[1] BioArctic and Lecanemab: A New Era in Alzheimer's [https://www.ainvest.com/news/bioarctic-lecanembi-era-alzheimer-treatment-market-implications-sustained-clinical-benefits-subcutaneous-innovation-2507][2] Alzheimer's disease market expected to reach $19.3bn [https://www.clinicaltrialsarena.com/analyst-comment/alzheimers-disease-market-8mm-2033/][3] FDA Approval of Leqembi Subcutaneous Formulation [https://www.morningstarMORN--.com/news/pr-newswire/20250829dc62435/fda-approval-of-leqembi-subcutaneous-formulation-charts-path-to-combination-therapies-for-alzheimers-disease][4] Eisai secures FDA green light for Leqembi autoinjector [https://www.fiercepharma.com/pharma/eisai-secures-highly-anticipated-fda-green-light-leqembi-iqlik-autoinjector-teeing-home]

Comentarios
Aún no hay comentarios