Eisai’s Dual Orexin-Targeting Pipeline: A Game Changer in Sleep Disorder Therapeutics
In the evolving landscape of neurological therapeutics, few companies have demonstrated the strategic foresight and scientific rigor of Eisai (TSE:4523). The Japanese pharmaceutical giant’s dual orexin-targeting pipeline—comprising the novel orexin 2 receptor agonist E2086 and the established orexin receptor antagonist lemborexant (DAYVIGO)—positions it to address a high-unmet-need segment of sleep disorders while capitalizing on a market projected to grow significantly over the next decade. This analysis evaluates the clinical, commercial, and investment implications of Eisai’s orexin-centric strategy, emphasizing its potential to redefine treatment paradigms and deliver long-term value.
E2086: A Novel Agonist for Narcolepsy, With Promising Phase Ib Data
Eisai’s E2086, presented at the World Sleep 2025 conference, represents a breakthrough in orexin-based therapeutics for narcolepsy type 1 (NT1). According to a report by Yahoo Finance, the Phase Ib trial (NCT06462404) demonstrated that once-daily E2086 significantly improved daytime wakefulness compared to both placebo and modafinil, the current standard of care [1]. Key metrics included enhanced sleep latency (as measured by the Maintenance of Wakefulness Test) and improved alertness (Karolinska Sleepiness Scale). Notably, treatment-emergent adverse events were predominantly mild to moderate, with no serious safety concerns reported [1].
This data underscores E2086’s potential to address a critical unmet need: NT1 affects approximately 1 in 2,000 individuals globally, yet existing treatments often fall short in efficacy or tolerability. As a selective orexin 2 receptor agonist, E2086 leverages Eisai’s deep understanding of the orexin system—a pathway central to regulating wakefulness—while building on the success of its orexin receptor antagonist, DAYVIGO. The compound’s differentiated mechanism and favorable safety profile could position it as a first-line therapy for NT1, with potential expansion into other hypersomnolence disorders.
Lemborexant (DAYVIGO): Sustained Market Growth and Global Expansion
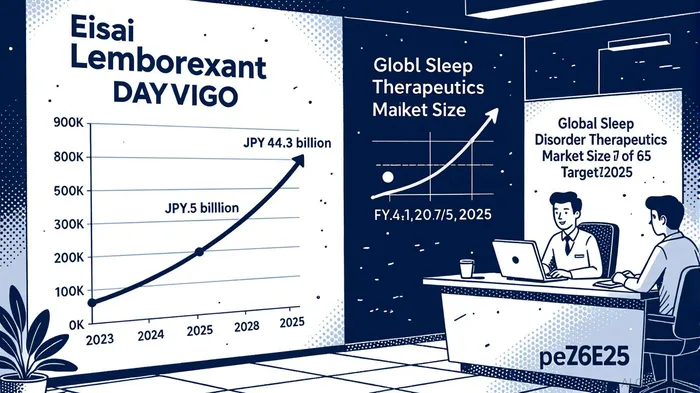
While E2086 represents Eisai’s future, lemborexant (DAYVIGO) has already established itself as a cornerstone of its present. The drug, approved for insomnia with sleep onset and maintenance issues, has seen robust adoption, particularly in the U.S. and now China, where it was launched in August 2025 [4]. Financial data from Eisai’s Q1 2025 earnings call reveals that lemborexant contributed JPY 16.9 billion to the company’s 3Ls (LENVIMA, LEQEMBI, and DAYVIGO) revenue of JPY 120.7 billion, with global sales reaching JPY 44.3 billion—exceeding initial guidance [3].
The U.S. market, in particular, is entering a “demand expansion phase,” driven by Eisai’s targeted marketing and the drug’s growing acceptance among clinicians [2]. With FY2025 revenue guidance set at JPY 76.5 billion for lemborexant, the compound is on track to become a blockbuster, fueled by its dual mechanism of action (orexin receptor antagonism and histamine H1 receptor antagonism) and favorable side-effect profile compared to traditional hypnotics like benzodiazepines.
Synergy and Long-Term Investment Potential
Eisai’s dual orexin-targeting pipeline creates a unique competitive moat. While lemborexant addresses insomnia—a market valued at over $5 billion globally—E2086 targets narcolepsy, a niche but high-margin segment. This diversification reduces exposure to market saturation in any single indication while leveraging Eisai’s proprietary orexin platform. Furthermore, the company’s in-house R&D capabilities and global commercial infrastructure (e.g., recent expansion into China) enhance its ability to scale both products effectively.
From an investment perspective, Eisai’s focus on unmet medical needs aligns with broader industry trends. Sleep disorders, often underdiagnosed and undertreated, are gaining attention as comorbidities with conditions like Alzheimer’s and cardiovascular disease become better understood. Eisai’s orexin-based therapies are well-positioned to benefit from this paradigm shift, particularly as E2086 advances through later-stage trials and lemborexant expands into emerging markets.
Risk Considerations and Strategic Outlook
Investors should remain mindful of potential risks, including clinical trial delays for E2086, regulatory hurdles in new markets, and competition from generic alternatives or novel therapies. However, Eisai’s track record in navigating complex regulatory environments—evidenced by the successful launch of LEQEMBI for Alzheimer’s—suggests a capacity to mitigate these challenges. Additionally, the company’s emphasis on innovation (e.g., orexin agonist/antagonist duality) provides a buffer against commoditization.
Conclusion
Eisai’s dual orexin-targeting pipeline exemplifies the intersection of scientific innovation and commercial pragmatism. With E2086 demonstrating robust Phase Ib results and lemborexant achieving blockbuster status, the company is poised to dominate a segment of the sleep disorder market characterized by high unmet needs and limited therapeutic alternatives. For long-term investors, Eisai offers a compelling case: a diversified, high-margin portfolio anchored by proprietary science and a clear path to global expansion.
**Source:[1] Eisai Presents Clinical Study Results of Novel Orexin 2 Receptor Agonist E2086 at World Sleep 2025, [https://finance.yahoo.com/news/eisai-presents-clinical-study-results-040100822.html][2] Global Lekambi sales reached ¥44.3B, exceeding guidance. FY2025 guidance targets ¥76.5B in Lekambi revenue, with U.S. entering the demand expansion phase., [https://finance.yahoo.com/quote/ESAIY/earnings/ESAIY-Q4-2025-earnings_call-304306.html/][3] Pharmaceutical business segment expansion due to growth in the 3Ls comprising LENVIMA, LEQEMBI, and lemborexant or DAYVIGO to 119% of the previous year., [https://www.alphaspread.com/security/tse/4523/investor-relations/earnings-call/q1-2026][4] Eisai Launches In-House Developed Anti-Insomnia Drug DAYVIGO(R) (Lemborexant) in China, [https://www.jcnnewswire.com/CompanyNews/102077/3]



Comentarios
Aún no hay comentarios