Delcath Systems' CHOPIN Phase 2 Trial and Its Implications for Liver-Directed Cancer Therapies
The oncology landscape is witnessing a seismic shift in the treatment of liver metastases, and Delcath SystemsDCTH-- (DCTH) is at the forefront of this revolution. With its CHOPIN Phase 2 trial set to debut at the 2025 European Society for Medical Oncology (ESMO) Annual Congress on October 18[1], the company is poised to redefine the standard of care for metastatic uveal melanoma (mUM) patients with liver involvement. This trial, which combines Delcath's CHEMOSAT® Hepatic Delivery System with the immunotherapy duo ipilimumab and nivolumab, has already shown groundbreaking results in its Phase 1b portion, . These figures not only outperform existing systemic therapies but also underscore the growing clinical momentum behind liver-directed approaches in oncology.
Clinical Momentum: A New Paradigm for mUM Treatment
Uveal melanoma, a rare but aggressive form of eye cancer, has long been a therapeutic challenge due to its high propensity for liver metastases and limited response to systemic treatments. Traditional systemic therapies for mUM have yielded dismal outcomes, . Delcath's , approved in 2024 as the first liver-directed therapy for mUM, . However, the CHOPIN trial aims to push these boundaries further by integrating immunotherapy into the equation.
The Phase 1b data from CHOPIN is nothing short of transformative. , . More remarkably, three of four patients who experienced disease progression continued treatment with repeated PHP cycles, suggesting durable clinical benefits. , . These results position Delcath's combination approach as a potential game-changer, .
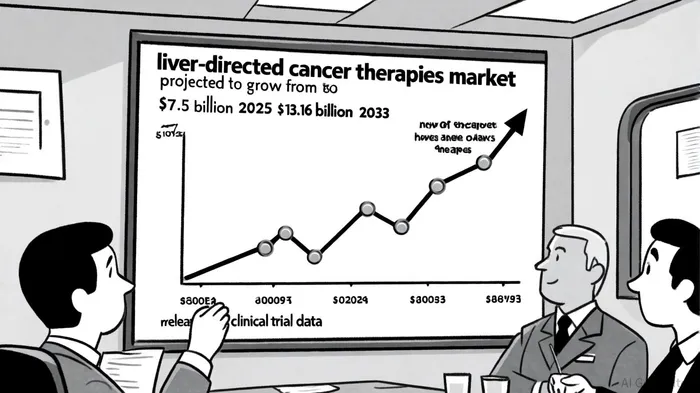
Market Opportunity: A $13.16 Billion Horizon by 2033
The liver-directed cancer therapies market is primed for explosive growth, driven by the rising incidence of (HCC) and advancements in targeted and immunotherapies. According to a report by Grand View Research, , . North America and the Asia-Pacific region are expected to dominate this expansion, fueled by aging populations, rising obesity rates, and improved healthcare infrastructure[6].
Within this broader market, the uveal melanoma niche is equally compelling. The uveal melanoma treatment market, , . While systemic therapies like tebentafusp (approved in 2024 for HLA-A*02:01-positive patients) have made strides, liver-directed therapies remain the cornerstone for patients with hepatic metastases[5]. Delcath's HEPZATO KIT currently holds a unique position as the only FDA-approved liver-directed therapy for mUM, . The CHOPIN trial's potential to further enhance these outcomes could solidify Delcath's leadership in this niche.
Competitive Landscape: Navigating a Crowded but Fragmented Field
Delcath faces competition from both systemic and locoregional therapies. On the systemic front, has emerged as a breakthrough, . However, its applicability is limited to a subset of patients, leaving a significant unmet need for broader solutions. Meanwhile, liver-directed competitors remain sparse. (PHP) and radioembolization are the primary alternatives, but neither has demonstrated the robust response rates seen in Delcath's trials[4].
The company's ability to combine PHP with immunotherapy could create a durable moat. As noted in a 2025 study, . This dual-action approach aligns with the industry's shift toward combination therapies, a trend exemplified by the success of Bristol-Myers Squibb's Opdivo and Merck's Keytruda in other oncology indications[6].
Investment Thesis: A High-Conviction Play in a High-Growth Niche
Delcath's CHOPIN trial represents more than a clinical milestone—it's a catalyst for redefining the value proposition of liver-directed therapies. With the ESMO presentation scheduled just weeks away, investors should closely monitor the Phase 2 data, particularly metrics like overall survival, durability of response, and safety profile. A positive readout could accelerate regulatory approvals and drive adoption in a market where treatment options remain scarce.
From a valuation perspective, . The company's proprietary CHEMOSAT platform, coupled with a robust pipeline of investigator-initiated trials, . For investors seeking exposure to the intersection of interventional oncology and immunotherapy, Delcath offers a compelling, high-conviction opportunity.

Comentarios
Aún no hay comentarios