Crinetics Pharmaceuticals and the Redefinition of Hormone Therapy: Unlocking Shareholder Value Through Innovation in Rare Adrenal Disease Treatment
The pharmaceutical industry's pursuit of precision medicine has long focused on addressing unmet needs in rare diseases, where innovation can yield outsized returns. CrineticsCRNX-- Pharmaceuticals' experimental treatment for congenital adrenal hyperplasia (CAH), atumelnant, represents a compelling case study in this paradigm. By targeting a genetic disorder with limited therapeutic options, the company is not only advancing a novel mechanism of action but also positioning itself to redefine hormone therapy for adrenal diseases. For investors, the question is whether this scientific breakthrough can translate into durable shareholder value-a proposition that hinges on clinical success, regulatory incentives, and market dynamics.
A Novel Approach to a Complex Disorder
CAH, a rare genetic condition affecting cortisol synthesis, imposes significant burdens on patients, including hormonal imbalances, adrenal hyperplasia, and complications such as infertility and growth impairment. Current standard-of-care treatments, primarily glucocorticoid and mineralocorticoid replacement therapies, aim to suppress overactive adrenal androgen production but often fall short in achieving optimal disease control. These therapies require meticulous dose adjustments and are associated with side effects like metabolic disturbances and growth suppression, underscoring an urgent need for alternatives according to clinical data.
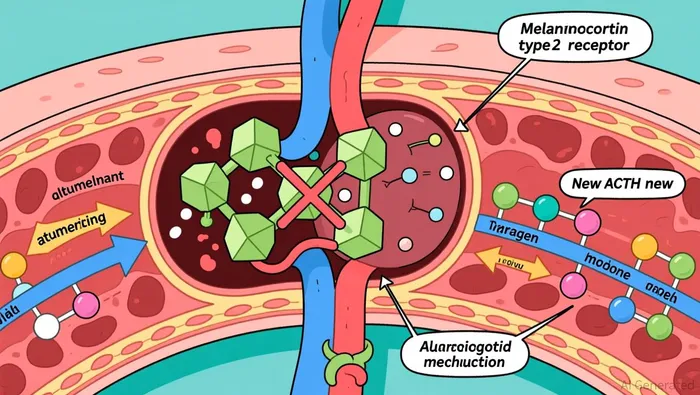 Atumelnant, a once-daily oral adrenocorticotropic hormone (ACTH) receptor antagonist, offers a mechanistically distinct solution. By selectively blocking the melanocortin type 2 receptor (MC2R) on adrenal glands, it interrupts the pathological overproduction of androgens like androstenedione (A4) and 17-hydroxyprogesterone (17-OHP) without systemic immunosuppression as research shows. Phase 2 trial results demonstrated an 80% mean reduction in A4 levels, alongside clinical improvements such as the resumption of menses in female participants and reductions in adrenal size according to the trial results. These outcomes suggest atumelnant could address both biochemical and symptomatic aspects of CAH, potentially reducing the need for lifelong hormone replacement and its associated complications.
Atumelnant, a once-daily oral adrenocorticotropic hormone (ACTH) receptor antagonist, offers a mechanistically distinct solution. By selectively blocking the melanocortin type 2 receptor (MC2R) on adrenal glands, it interrupts the pathological overproduction of androgens like androstenedione (A4) and 17-hydroxyprogesterone (17-OHP) without systemic immunosuppression as research shows. Phase 2 trial results demonstrated an 80% mean reduction in A4 levels, alongside clinical improvements such as the resumption of menses in female participants and reductions in adrenal size according to the trial results. These outcomes suggest atumelnant could address both biochemical and symptomatic aspects of CAH, potentially reducing the need for lifelong hormone replacement and its associated complications.
Strategic Advantages and Market Potential
Crinetics' development strategy is bolstered by regulatory tailwinds. The FDA's Orphan Drug Designation for atumelnant in classic CAH provides incentives such as tax credits, expedited review, and seven years of market exclusivity upon approval according to official statements. These benefits are critical in a niche market where patient populations are small but treatment costs are high. With global CAH prevalence estimated at 1 in 10,000 to 1 in 18,000 births according to clinical estimates, the commercial potential for a disease-modifying therapy is substantial, particularly if atumelnant gains approval for both adult and pediatric indications.
The company's clinical pipeline further strengthens its position. The ongoing Phase 3 CALM-CAH trial in adults and the planned Phase 2/3 BALANCE-CAH trial in pediatric patients according to the company's announcement are pivotal in establishing long-term safety and efficacy. Success in these trials could differentiate atumelnant from existing therapies and establish it as a first-line treatment, potentially displacing conventional glucocorticoids. For investors, this represents a dual opportunity: capturing market share in a currently underserved space and leveraging the drug's intellectual property to deter competition.
Risks and Considerations
Despite its promise, atumelnant's path to commercialization is not without risks. Clinical trials in rare diseases often face challenges in patient recruitment and endpoint definition, while regulatory approval hinges on demonstrating not only efficacy but also cost-effectiveness relative to existing treatments. Additionally, the absence of direct competitors-while advantageous-means Crinetics must educate payers and providers on the drug's value proposition, a process that could delay adoption.
However, the company's focus on a clear unmet need and its alignment with orphan drug incentives mitigate some of these risks. The rarity of CAH also reduces the likelihood of near-term competition, allowing Crinetics to consolidate its market position before broader adrenal disease therapies emerge.
Conclusion: A Catalyst for Shareholder Value
For Crinetics, atumelnant embodies more than a scientific innovation-it represents a strategic pivot toward high-margin, specialized therapeutics. If the drug gains approval, its potential to redefine hormone therapy for adrenal diseases could unlock significant shareholder value through revenue growth, partnerships, or even acquisition interest from larger pharma players seeking to expand their rare disease portfolios. In an industry increasingly defined by precision and personalization, Crinetics' progress in CAH underscores the rewards of targeting niche markets with transformative solutions.

Comentarios
Aún no hay comentarios