Compass Therapeutics (CMPX): A High-Potential Oncology Biotech on the Cusp of Clinical and Strategic Momentum
In the crowded and high-stakes world of oncology biotech, Compass TherapeuticsCMPX-- (NASDAQ:CMPX) stands out as a company poised to redefine therapeutic paradigms with its bispecific antibody pipeline. With a focus on DLL4 and VEGF-A pathways—key drivers of tumor angiogenesis and immune evasion—the firm is leveraging its scientific expertise to address unmet needs in aggressive cancers like biliary tract cancer. Recent clinical data, strategic partnerships, and capital-efficient execution position CMPXCMPX-- as a compelling investment opportunity for those seeking exposure to innovation in immuno-oncology.
Pipeline Differentiation: Bispecifics as a Game Changer
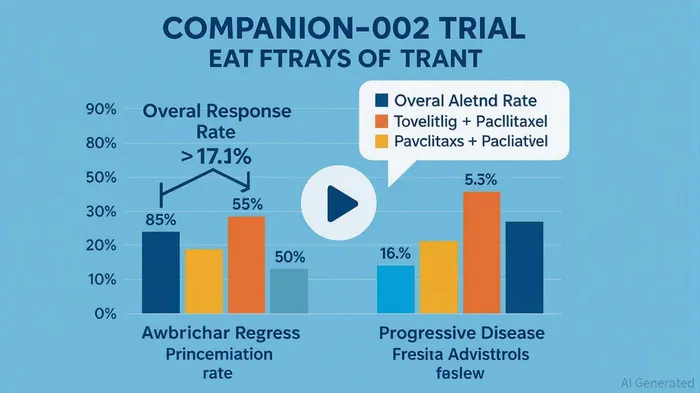
Compass's lead candidate, tovecimig (CTX-009), is a DLL4 x VEGF-A bispecific antibody that has demonstrated robust clinical activity in second-line biliary tract cancer (BTC). The Phase 2/3 COMPANION-002 trial, which compared tovecimig plus paclitaxel to paclitaxel alone, reported a 17.1% overall response rate (ORR) versus 5.3% in the control arm—a statistically significant and clinically meaningful improvement[3]. Additionally, the study observed a 16.2% progressive disease rate in the tovecimig arm versus 42.1% in the paclitaxel-only group[3]. These results, coupled with the FDA Fast Track designation granted in April 2024[3], underscore the drug's potential to become a standard of care in BTC, a disease with limited treatment options.
The bispecific antibody platform itself represents a strategic advantage. Unlike traditional monoclonal antibodies, bispecifics can simultaneously target two distinct pathways or cell types. In tovecimig's case, this dual inhibition of DLL4 (a Notch ligand critical for tumor angiogenesis) and VEGF-A (a key angiogenic driver) creates a synergistic effect that disrupts tumor blood supply while enhancing immune cell infiltration[1]. This mechanism is particularly relevant in BTC, where the tumor microenvironment is notoriously immunosuppressive.
Strategic Momentum: Expanding the Pipeline and Clinical Reach
Compass is not resting on its laurels. The company is advancing CTX-8371, another bispecific antibody targeting DLL4 and PD-L1, in a Phase 1 trial for post-checkpoint inhibitor settings[3]. Meanwhile, CTX-10726, a PD-1 x VEGF-A bispecific, is slated for IND submission in Q4 2025[3], further diversifying the pipeline. These programs reflect a disciplined approach to innovation, with each candidate addressing distinct oncology challenges while leveraging Compass's core expertise in angiogenesis and immunology.
The recent Morgan Stanley 23rd Annual Global Healthcare Conference presentation highlighted CEO Thomas Schuetz's emphasis on capital efficiency and pipeline breadth. Schuetz noted that Compass's three-drug clinical portfolio—two bispecifics and one monoclonal antibody—positions the company to generate multiple data readouts in 2025 and 2026[1]. Notably, the COMPANION-002 secondary endpoints, including overall survival and progression-free survival, are expected in Q1 2026[3], providing a critical inflection point for the stock.
Capital Efficiency: A Model for Sustainable Growth
Biotech investors often prioritize companies that can stretch their cash runway while advancing key programs. CompassCOMP-- Therapeutics ended Q2 2025 with $101 million in cash and marketable securities, sufficient to fund operations through 2027[3]. This financial flexibility is a testament to the company's lean operational structure and strategic focus on high-impact trials. For context, the COMPANION-002 trial enrolled 168 patients—a relatively modest number for a Phase 3 study—yet delivered robust data[3]. Such efficiency is rare in oncology, where large, expensive trials are the norm.
The company's capital allocation strategy is further bolstered by investor-friendly milestones. The upcoming Investigator-Sponsored Trial (IST) at MD Anderson Cancer Center, which is evaluating tovecimig in combination with gemcitabine, cisplatin, and durvalumab as a first-line therapy[3], could generate additional clinical validation without significant incremental costs. Similarly, the planned basket study of tovecimig in DLL4+ cancers (e.g., gastric, ovarian, and renal) is designed to maximize the drug's addressable market[3].
Strategic Positioning in the Biotech Ecosystem
Compass's approach aligns with broader industry trends. Bispecific antibodies are gaining traction as a next-generation modality, with companies like AmgenAMGN-- and Roche investing heavily in the space. However, Compass's focus on DLL4 and VEGF-A—two underexplored but highly synergistic targets—offers a unique value proposition. As noted by management during the Morgan StanleyMS-- presentation, the company's scientific foundation in angiogenesis and immunology provides a “rare combination of mechanistic clarity and clinical agility”[1].
Moreover, the investor events roadmap for 2025, including the September conference and Q2 2025 financial results disclosure[2], ensures continued visibility and engagement with the market. This transparency is critical for a clinical-stage biotech, as it helps manage expectations and build credibility with stakeholders.
Conclusion: A Biotech with Momentum and Vision
Compass Therapeutics is a rare blend of scientific innovation and operational discipline. With tovecimig's promising BTC data, a robust bispecific pipeline, and a capital-efficient model, the company is well-positioned to deliver outsized returns for investors. As the oncology landscape evolves, CMPX's focus on DLL4 and VEGF-A—two pillars of tumor biology—could redefine treatment paradigms and unlock significant value. For those seeking exposure to a high-conviction, low-capital biotech play, Compass Therapeutics is a name worth watching.

Comentarios
Aún no hay comentarios