Cognition Therapeutics Presents Promising Zervimesine Phase 2 Study Results
PorAinvest
jueves, 17 de julio de 2025, 8:39 pm ET1 min de lectura
CGTX--
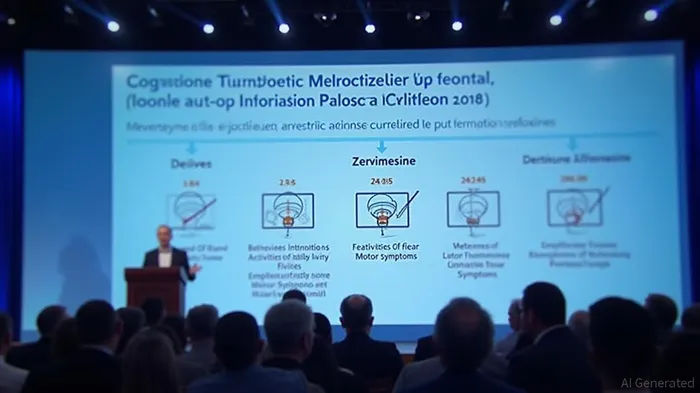
The study showed significant improvements in behavioral outcomes, activities of daily living, cognitive fluctuations, and motor symptoms among zervimesine-treated participants compared to placebo. Specifically, participants treated with zervimesine tested 86% better on behavioral outcomes (NPI 12), 52% on activities of daily living, 91% on cognitive fluctuations, and 62% on motor symptoms compared to placebo [2].
Dr. Galvin, the lead investigator, expressed optimism about zervimesine's potential in treating DLB symptoms. "The results of the Phase 2 SHIMMER study give hope to the millions of people living with DLB and their healthcare teams, who struggle to treat this complex disease," he stated. "My colleagues and I believe that there is great potential in a once-daily oral medication that slows disease progress while simultaneously reducing the severity and frequency of some of the most troublesome symptoms of DLB."
DLB is the second most common cause of dementia, affecting approximately 1.4 million Americans. Currently, no disease-modifying therapeutics are approved for DLB. The SHIMMER study was supported by a grant award from the National Institute on Aging of the National Institutes of Health (NIH) totaling approximately $30 million [1].
Cognition Therapeutics will present clinical efficacy results and new proteomic findings from both the SHIMMER and SHINE studies at AAIC. The SHINE study, which enrolled 153 adults with mild-to-moderate Alzheimer’s disease, showed that zervimesine treatment preserved cognitive and functional abilities better than placebo in participants with lower levels of p-Tau217 [2].
The SHIMMER study's results, along with the SHINE study's findings, suggest that zervimesine has broad neuroprotective properties. This is particularly encouraging for investors and financial professionals, as it indicates the potential for zervimesine to slow the progression of DLB and improve the lives of those suffering from this complex disease.
References:
[1] https://www.marketscreener.com/quote/stock/COGNITION-THERAPEUTICS-IN-127926318/news/Cognition-Therapeutics-Inc-Announces-Positive-Clinical-Data-from-Zervimesine-Phase-2-Study-in-Deme-50525294/
[2] https://www.biospace.com/press-releases/cognition-therapeutics-positive-clinical-data-from-zervimesine-ct1812-phase-2-study-in-dementia-with-lewy-bodies-dlb-will-be-presented-in-a-podium-presentation-at-aaic
Cognition Therapeutics will present data from its Phase 2 "SHIMMER" study of zervimesine in dementia with Lewy bodies at the Alzheimer's Association International Conference. Results show zervimesine-treated participants had significant improvements in behavioral outcomes, activities of daily living, cognitive fluctuations, and motor symptoms compared to placebo. The study's lead investigator believes zervimesine has great potential in treating DLB symptoms.
Cognition Therapeutics, Inc. is set to present data from its Phase 2 "SHIMMER" study of zervimesine (CT1812) in dementia with Lewy bodies (DLB) at the Alzheimer's Association International Conference (AAIC). The study, directed by James E. Galvin, MD, MPH, and conducted in collaboration with the Lewy Body Dementia Association (LBDA), enrolled 130 adults with mild-to-moderate DLB, who were randomized to receive either daily oral doses of zervimesine (100 mg or 300 mg) or placebo for six months [1].The study showed significant improvements in behavioral outcomes, activities of daily living, cognitive fluctuations, and motor symptoms among zervimesine-treated participants compared to placebo. Specifically, participants treated with zervimesine tested 86% better on behavioral outcomes (NPI 12), 52% on activities of daily living, 91% on cognitive fluctuations, and 62% on motor symptoms compared to placebo [2].
Dr. Galvin, the lead investigator, expressed optimism about zervimesine's potential in treating DLB symptoms. "The results of the Phase 2 SHIMMER study give hope to the millions of people living with DLB and their healthcare teams, who struggle to treat this complex disease," he stated. "My colleagues and I believe that there is great potential in a once-daily oral medication that slows disease progress while simultaneously reducing the severity and frequency of some of the most troublesome symptoms of DLB."
DLB is the second most common cause of dementia, affecting approximately 1.4 million Americans. Currently, no disease-modifying therapeutics are approved for DLB. The SHIMMER study was supported by a grant award from the National Institute on Aging of the National Institutes of Health (NIH) totaling approximately $30 million [1].
Cognition Therapeutics will present clinical efficacy results and new proteomic findings from both the SHIMMER and SHINE studies at AAIC. The SHINE study, which enrolled 153 adults with mild-to-moderate Alzheimer’s disease, showed that zervimesine treatment preserved cognitive and functional abilities better than placebo in participants with lower levels of p-Tau217 [2].
The SHIMMER study's results, along with the SHINE study's findings, suggest that zervimesine has broad neuroprotective properties. This is particularly encouraging for investors and financial professionals, as it indicates the potential for zervimesine to slow the progression of DLB and improve the lives of those suffering from this complex disease.
References:
[1] https://www.marketscreener.com/quote/stock/COGNITION-THERAPEUTICS-IN-127926318/news/Cognition-Therapeutics-Inc-Announces-Positive-Clinical-Data-from-Zervimesine-Phase-2-Study-in-Deme-50525294/
[2] https://www.biospace.com/press-releases/cognition-therapeutics-positive-clinical-data-from-zervimesine-ct1812-phase-2-study-in-dementia-with-lewy-bodies-dlb-will-be-presented-in-a-podium-presentation-at-aaic
Divulgación editorial y transparencia de la IA: Ainvest News utiliza tecnología avanzada de Modelos de Lenguaje Largo (LLM) para sintetizar y analizar datos de mercado en tiempo real. Para garantizar los más altos estándares de integridad, cada artículo se somete a un riguroso proceso de verificación con participación humana.
Mientras la IA asiste en el procesamiento de datos y la redacción inicial, un miembro editorial profesional de Ainvest revisa, verifica y aprueba de forma independiente todo el contenido para garantizar su precisión y cumplimiento con los estándares editoriales de Ainvest Fintech Inc. Esta supervisión humana está diseñada para mitigar las alucinaciones de la IA y garantizar el contexto financiero.
Advertencia sobre inversiones: Este contenido se proporciona únicamente con fines informativos y no constituye asesoramiento profesional de inversión, legal o financiero. Los mercados conllevan riesgos inherentes. Se recomienda a los usuarios que realicen una investigación independiente o consulten a un asesor financiero certificado antes de tomar cualquier decisión. Ainvest Fintech Inc. se exime de toda responsabilidad por las acciones tomadas con base en esta información. ¿Encontró un error? Reportar un problema

Comentarios
Aún no hay comentarios