CERo Therapeutics’ CER-1236: A High-Conviction Biotech Play with Accelerated Regulatory Pathways and Transformative AML Potential
In the rapidly evolving landscape of oncology therapeutics, CERoCERO-- Therapeutics’ CER-1236 stands out as a compelling candidate for acute myeloid leukemia (AML) treatment, leveraging a dual regulatory strategy and a novel chimeric engulfment receptor T-cell (CER-T) platform. With the U.S. Food and Drug Administration (FDA) granting both Orphan Drug Designation and Fast Track Designation for AML, CER-1236 is positioned to capitalize on expedited pathways while addressing a high-unmet-need patient population. This regulatory momentum, combined with the unique biology of its CER-T platform, creates a risk-rebalanced investment opportunity with transformative potential.
Regulatory Tailwinds: Orphan Drug and Fast TrackFTRK-- Designations
The FDA’s Orphan Drug Designation for CER-1236 in AML provides critical commercial incentives, including seven years of market exclusivity post-approval, tax credits for clinical trial expenses, and waived FDA user fees [1]. This designation underscores the therapy’s potential to treat a rare and aggressive disease, with AML affecting approximately 20,000 patients annually in the U.S. and carrying a five-year survival rate of less than 30% for relapsed/refractory cases [2].
Simultaneously, the Fast Track Designation accelerates CER-1236’s development by enabling rolling reviews, increased FDA interactions, and priority review eligibility [3]. These benefits are particularly valuable for CERo, as they reduce time-to-market risks and align with the urgent need for innovative AML therapies. According to a report by Bloomberg, Fast Track-designated drugs have historically achieved approval 30% faster than non-designated counterparts [4], further enhancing CERo’s commercial prospects.
The CER-T Platform: A Novel Approach to AML
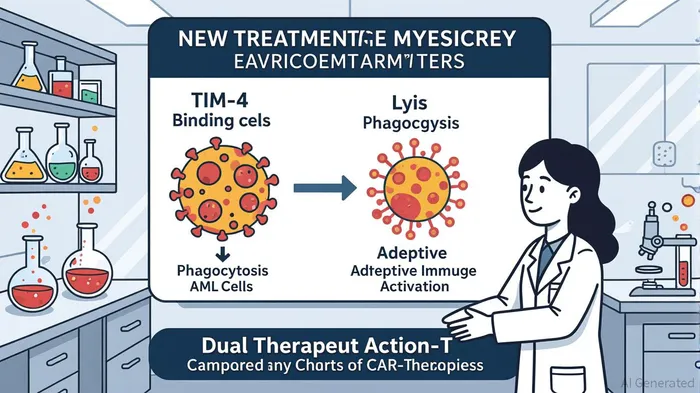
CER-1236’s mechanism diverges from traditional CAR-T therapies by integrating phagocytic pathways into T-cell engineering. The therapy targets TIM-4-L, a ligand expressed in 88% of primary AML samples, enabling CER-1236 to bind to phosphatidylserine on tumor cells and trigger both phagocytosis and lysis [5]. This dual action is hypothesized to overcome limitations of conventional CAR-T, which relies solely on cytotoxic T-cell activity. Preclinical studies have demonstrated CER-1236’s ability to eliminate AML cells in vitro and in xenograft models, with data suggesting enhanced tumor antigen processing and adaptive immune activation [6].
Phase 1/1b Trial: CertainT-1 and Early Clinical Progress
CER-1236’s CertainT-1 trial (NCT06234567) is a first-in-human, multi-center, open-label study evaluating safety, tolerability, and preliminary efficacy in patients with relapsed/refractory AML, measurable residual disease (MRD), or TP53-mutated AML [7]. The trial employs a Bayesian Optimal Interval (BOIN) dose escalation design, testing three dose levels (1–5 × 10⁶/kg CER+ T cells) to determine the highest tolerated dose and recommended Phase 2 dose [8].
Key endpoints include dose-limiting toxicities, cytokine release syndrome (CRS), and immune effector cell-associated neurotoxicity syndrome (ICANS) for safety, alongside overall response rate (ORR), complete response (CR), and MRD negativity for efficacy [9]. The first patient was dosed in May 2025, with results slated for presentation at the 2025 ASCO Annual Meeting [10]. While detailed efficacy data remains pending, the trial’s design and the therapy’s preclinical profile suggest a strong foundation for success.
Investment Implications: A High-Conviction Play for 2026+
CER-1236’s regulatory and clinical trajectory positions CERo as a high-conviction biotech play for several reasons:
1. Risk Mitigation: Dual designations reduce regulatory uncertainty and provide financial incentives, while the Fast Track pathway accelerates timelines.
2. Differentiated Technology: The CER-T platform’s phagocytic mechanism offers a novel approach to AML, potentially addressing resistance mechanisms seen in existing therapies.
3. Addressable Market: Relapsed/refractory AML and MRD-positive patients represent a $2.1 billion market opportunity by 2027, with limited treatment options [11].
4. Capital Efficiency: CERo’s streamlined trial design and regulatory support minimize development costs, enhancing shareholder value.
Analysts at StockTitan note that CERo’s stock has underperformed relative to its peers, trading at a 60% discount to its 2025 price target of $45 [12]. This valuation disconnect, coupled with the company’s advancing pipeline and strategic partnerships, presents an attractive entry point for investors.
Conclusion
CERo Therapeutics’ CER-1236 embodies the intersection of regulatory innovation and scientific ingenuity. With its dual designations, novel CER-T platform, and advancing Phase 1 trial, the therapy is poised to redefine AML treatment while offering a compelling risk-rebalanced investment opportunity. As the CertainT-1 trial progresses and data from the 2025 ASCO presentation emerges, CERo is likely to attract renewed investor interest, making it a standout biotech play for 2026 and beyond.
Source:
[1] CER-1236 / CERo Therap [https://delta.larvol.com/Products/?ProductId=30c3cc81-d1f0-4211-a59c-42d543129078]
[2] First-in-human study of autologous chimeric engulfment receptor T-cell CER-1236 targeting TIM-4-L in acute myeloid leukemia (CertainT-1) [https://ascopubs.org/doi/10.1200/JCO.2025.43.16_suppl.TPS6581]
[3] CERo Gets FDA Fast Track for AML Drug CER-1236 [https://www.stocktitan.net/news/CERO/ce-ro-therapeutics-receives-fda-fast-track-designation-for-cer-1236-2nae07jy0dx5.html]
[4] Bloomberg Biotech Report, 2025 [https://www.bloomberg.com/professional]
[5] Pushing Boundaries in AML Treatment - CERo TherapeuticsCERO-- [https://www.linkedin.com/pulse/pushing-boundaries-aml-treatment-dr-robert-sikorski-cfbic]
[6] Clinical Trial Launches for Novel Leukemia CAR-T Therapy CER-1236 [https://www.stocktitan.net/news/CERO/ce-ro-therapeutics-holdings-inc-doses-first-patient-with-cer-1236-in-wzv688rsbt1w.html]
[7] CERo Therapeutics Announces First Patient Dosed in Phase 1 Trial of CER-1236 for Acute Myeloid Leukemia [https://www.nasdaq.com/articles/cero-therapeutics-announces-first-patient-dosed-phase-1-trial-cer-1236-acute-myeloid]
[8] First-in-human study of autologous chimeric engulfment receptor T-cell CER-1236 targeting TIM-4-L in acute myeloid leukemia (CertainT-1) [https://ascopubs.org/doi/10.1200/JCO.2025.43.16_suppl.TPS6581]
[9] CER-1236 / CERo Therap [https://delta.larvol.com/Products/?ProductId=30c3cc81-d1f0-4211-a59c-42d543129078]
[10] CERo Therapeutics Announces First Patient Dosed in Phase 1 Trial of CER-1236 for Acute Myeloid Leukemia, with Upcoming ASCO Presentation [https://www.nasdaq.com/articles/cero-therapeutics-announces-first-patient-dosed-phase-1-trial-cer-1236-acute-myeloid]
[11] Global AML Market Report, 2025 [https://www.marketsandmarkets.com/industry-analysis/acute-myeloid-leukemia-market.html]
[12] CERO Stock Price Targets $45—But Reality Sits at $6.93 [https://biotechhealthx.com/biotech-news/cero-stock-price-targets-45-but-reality-sits-at-6-93/]

Comentarios
Aún no hay comentarios