BioMarin Pharmaceutical's Q3 2025 Performance: Navigating R&D Costs and Gene Therapy Competition
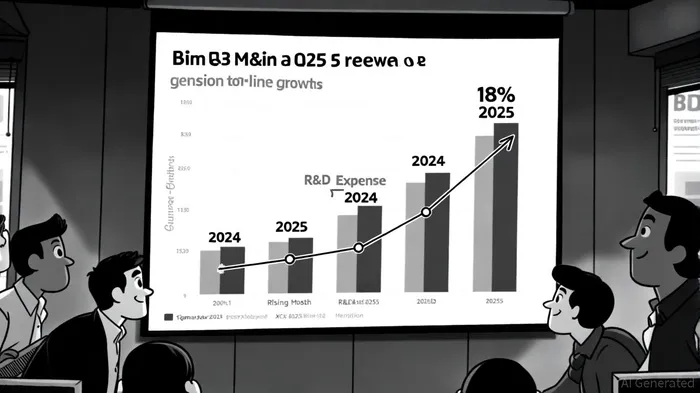
BioMarin Pharmaceutical's Q3 2025 financial results reflect a resilient top line but underscore the growing strain of R&D expenditures, raising critical questions about its long-term growth trajectory in an increasingly competitive gene therapy landscape. According to CapyFin's Q3 2025 earnings report, the company's estimated revenue for Q3 2025 reached $790 million, representing an 11.8% year-over-year increase, with an earnings per share (EPS) of $0.77. This follows a robust Q1 2025 performance, where revenue surged 15% to $745 million, driven by a 40% year-over-year rise in VOXZOGO sales, bolstered by large government orders as noted in a BioSpace article. However, an 18% increase in R&D expenses during Q3 2025 has begun to erode profitability margins, per a FierceBiotech report.
Strategic R&D Realignment: Focus and Constraints
Under new leadership, BioMarinBMRN-- has streamlined its pipeline to three core programs: BMN 333 (a CNP candidate for growth disorders), BMN 349 (an oral therapy for alpha-1 antitrypsin deficiency), and BMN 351 (a Duchenne muscular dystrophy oligonucleotide), as reported by FierceBiotech. This realignment aims to reduce R&D costs and accelerate development, but the company faces significant hurdles. For instance, BMN 333, which showed promising pharmacokinetic data in healthy volunteers, is slated for a pivotal Phase 2/3 trial in mid-2026, with a potential 2030 launch (FierceBiotech). Meanwhile, BMN 351's initial clinical data is expected by year-end 2025, though its long-term commercial viability remains unproven.
The company's flagship gene therapy, ROCTAVIAN (valoctocogene roxaparvovec), for hemophilia A, exemplifies both promise and peril. Despite demonstrating sustained efficacy over five years in its five-year Phase 3 results, its commercial adoption has been sluggish, with only five patients treated as of Q2 2025 and $7 million in revenue (BioSpace). BioMarin has scaled back Roctavian's commercial footprint to the U.S., Germany, and Italy-markets with established reimbursement-to cut annual spending to $60 million (CapyFin). Analysts question whether this strategy can turn the therapy profitable by 2025, particularly as Novo Nordisk's Mim8 (a subcutaneous bispecific antibody in Phase 3) and Roche's ALTUVIIIO (an extended half-life factor VIII therapy) gain traction, according to a Hemophilia Drug Pipeline Report.
Competitive Pressures and Market Dynamics
The hemophilia A space is witnessing a paradigm shift toward non-factor therapies and one-time gene treatments. Novo Nordisk's Mim8, with its flexible dosing intervals and subcutaneous administration, is positioned to disrupt traditional factor replacement markets (Hemophilia Drug Pipeline Report). Similarly, Alnylam Pharmaceuticals' Qfitlia, an RNAi therapy approved in March 2025, has introduced a novel mechanism of action, further fragmenting BioMarin's market share (BioSpace). These advancements highlight the industry's pivot toward curative solutions but also amplify BioMarin's challenges in justifying Roctavian's high price tag and limited patient access.
Despite these headwinds, BioMarin's management remains optimistic. The company has outlined a long-term revenue growth plan targeting $4 billion by 2027, leveraging its expertise in rare diseases and enzyme therapies (BioSpace). However, this ambition hinges on the success of its three prioritized R&D programs and the ability to navigate reimbursement barriers for gene therapies.
Long-Term Outlook: Balancing Innovation and Profitability
BioMarin's Q3 2025 results underscore a company at a crossroads. While its revenue growth and rare disease franchise provide a stable foundation, the rising R&D costs and competitive pressures in gene therapy pose existential risks. The company's decision to divest non-core assets, including Roctavian, remains a wildcard that could reshape its financial profile. For investors, the key will be monitoring the Phase 2/3 data for BMN 333 and the commercial traction of BMN 351, alongside the broader market's adoption of next-gen therapies.
In the short term, BioMarin's focus on cost discipline and pipeline streamlining offers a path to profitability. Yet, in the long term, the company's ability to innovate in a rapidly evolving gene therapy landscape-and differentiate itself from rivals like Novo Nordisk and Roche-will determine whether it can sustain its growth trajectory.

Comentarios
Aún no hay comentarios