BioAtla's Oz-V SPV Deal: A Strategic Financing Masterstroke in Oncology and Longevity
In an era where biotech innovation increasingly hinges on financial agility, BioAtla's recent $40 million Special Purpose Vehicle (SPV) deal for ozuriftamab vedotin (Oz-V) stands out as a model of strategic value creation. By leveraging an asset-specific financing structure and forging cross-sector partnerships, the company has not only secured critical capital for its lead oncology candidate but also positioned itself at the intersection of oncology and longevity research. This analysis examines how the Oz-V SPV deal exemplifies a forward-thinking approach to capital allocation and therapeutic diversification.
The Oz-V SPV Deal: A Tailored Financing Framework
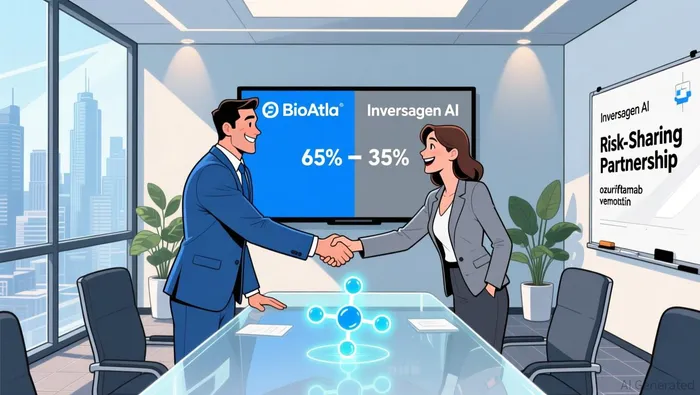
The Oz-V SPV, announced on December 31, 2025, is structured to advance the drug into a Phase 3 trial for second-line or later oropharyngeal squamous cell carcinoma (OPSCC). Under the agreement, BioAtlaBCAB-- receives an initial $5 million for operational and clinical expenses, with the remaining $35 million contingent on the initiation of the Phase 3 trial in Q1 2026 according to the announcement. This staged funding model aligns capital deployment with clinical milestones, reducing dilution for existing shareholders while ensuring resources are available for trial execution.
Crucially, the SPV's ownership structure reflects a balanced risk-sharing framework. Inversagen AI, LLC-backed by GATC Health Corp. and Inversagen LLC-holds a 35% stake in Oz-V, while BioAtla retains 65% ownership across all solid tumor indications as detailed in the announcement. This arrangement preserves BioAtla's control over its core asset while attracting strategic partners with expertise in AI-driven drug development. Such partnerships are increasingly vital in an industry where computational tools are reshaping therapeutic discovery.
Strategic Rationale: Equity Preservation and Accelerated Development
The SPV's primary strategic advantage lies in its ability to bypass traditional equity raises, which often dilute shareholder value. By securing non-dilutive capital, BioAtla mitigates the financial risks associated with prolonged clinical trials. According to BioAtla's investor relations team, this approach "maximizes equity value while advancing a high-potential asset with a clear regulatory pathway."
The decision to pursue a Phase 3 trial for OPSCC is also strategically sound. Oz-V has already received FDA Fast Track Designation for recurrent or metastatic squamous cell carcinoma of the head and neck, a designation that expedites regulatory review and increases the likelihood of accelerated approval. With patient enrollment slated to begin in early 2026, the trial's timeline is optimized to capitalize on this regulatory advantage.
Cross-Sector Expansion: Bridging Oncology and Longevity
Beyond its immediate oncology focus, the SPV deal unlocks opportunities in the longevity sector. The collaboration with Inversagen AI extends beyond Oz-V, with both parties agreeing to co-develop Conditionally Active Biologic (CAB) senolytic therapies. Senolytics-drugs that target senescent cells linked to aging-related diseases-represent a burgeoning field with significant unmet medical needs. By retaining rights to cancer therapeutic applications of CAB technologies, BioAtla diversifies its pipeline while leveraging Inversagen's AI capabilities to accelerate discovery.
 This cross-sector expansion is emblematic of a broader trend in biotech: the convergence of oncology and aging research. As noted by industry analysts, therapies that address both cancer and age-related degeneration are poised to capture substantial market share, given the rising global prevalence of these conditions. BioAtla's dual focus on OPSCC and senolytics positions it to benefit from this dual demand.
This cross-sector expansion is emblematic of a broader trend in biotech: the convergence of oncology and aging research. As noted by industry analysts, therapies that address both cancer and age-related degeneration are poised to capture substantial market share, given the rising global prevalence of these conditions. BioAtla's dual focus on OPSCC and senolytics positions it to benefit from this dual demand.
Investment Implications: Balancing Risks and Rewards
While the SPV deal is a clear win for capital efficiency, investors must weigh the inherent risks of Phase 3 trials. Clinical failures remain common, and the $35 million second tranche is contingent on successful trial initiation. However, the upfront funding and ownership structure provide a buffer against such uncertainties.

Comentarios
Aún no hay comentarios