Can BEAM's Base-Editing Pipeline Outperform CRISPR Therapeutics and Intellia in the Gene Editing Space?
The gene-editing landscape in 2025 is defined by a race to deliver precision, safety, and commercial viability. Among the key players, Beam Therapeutics, CRISPR Therapeutics, and Intellia Therapeutics represent distinct approaches to genetic medicine. While CRISPR TherapeuticsCRSP-- and IntelliaNTLA-- have made strides with CRISPR-Cas9 and in vivo delivery systems, Beam's base-editing pipeline offers a compelling alternative. This analysis evaluates whether Beam's technology and strategy can outperform its rivals in the long term, focusing on competitive differentiation and value creation in precision genetic medicine.
Competitive Differentiation: Precision and Safety as a Strategic Edge
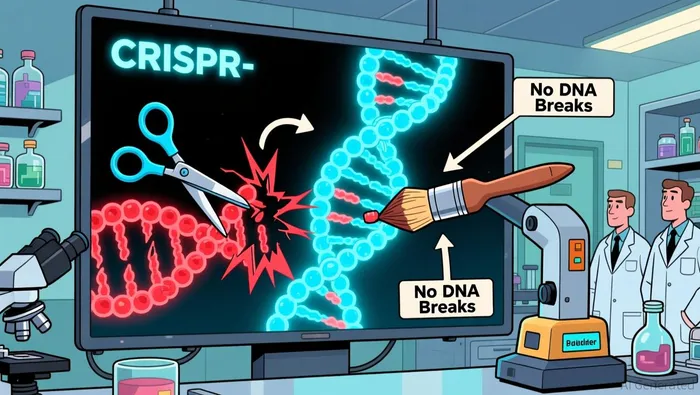
Beam's core advantage lies in its base-editing technology, which enables single-nucleotide modifications without inducing double-stranded DNA breaks. This approach theoretically reduces off-target effects compared to traditional CRISPR-Cas9 methods, which rely on DNA cleavage and repair mechanisms. For example, Beam's lead candidate, risto-cel (BEAM-101), for sickle cell disease (SCD) has demonstrated a mean fetal hemoglobin (HbF) induction of over 60% and a reduction of hemoglobin S (HbS) to below 40% in 31 patients, with sustained anemia resolution for up to 20 months according to clinical data. These results, presented at the 2025 ASH Annual Meeting, underscore the therapy's efficacy while aligning with the safety profile of busulfan conditioning and autologous stem cell transplantation as reported.
In contrast, CRISPR Therapeutics' Casgevy, the first CRISPR-based therapy approved for SCD and beta-thalassemia, relies on CRISPR-Cas9 to disrupt the BCL11A gene. While effective, its mechanism involves DNA cleavage, which could raise long-term safety concerns for investors. Intellia's in vivo candidates, such as lonvo-z for hereditary angioedema, leverage lipid nanoparticle (LNP) delivery but face challenges in achieving precise edits without off-target activity as noted in market analysis. Beam's base-editing approach, by avoiding DNA breaks, may position it as a safer alternative in diseases where precision is critical.
Clinical Progress and Regulatory Momentum
Beam's pipeline is advancing rapidly, with BEAM-101 in phase I/II trials for SCD and BEAM-302 for alpha-1 antitrypsin deficiency (AATD) showing early promise. The FDA's Regenerative Medicine Advanced Therapy (RMAT) and Orphan Drug designations for BEAM-101 in 2025 highlight its potential to address unmet needs in rare diseases. Meanwhile, CRISPR Therapeutics has already secured regulatory approvals for Casgevy, giving it a commercial head start. However, Beam's focus on ex vivo therapies-where cells are edited outside the body before reinfusion-may offer greater control over editing outcomes compared to in vivo approaches, which face delivery and dosing challenges as detailed in financial reports.
Intellia's HAELO phase III trial for lonvo-z, expected to report top-line data by mid-2026, represents a critical milestone. Yet, Beam's ex vivo base-editing strategy for SCD has already demonstrated durable responses in a larger patient cohort, potentially accelerating its path to approval according to company announcements.
Market Dynamics and Financial Resilience
The global base-editing market is projected to grow at a 15.4% CAGR from 2024 to 2030, reaching $741.1 million by 2030 according to market analysis. This growth is driven by demand for therapies with reduced off-target risks, particularly in monogenic diseases. Beam's financial position supports its long-term ambitions: as of Q3 2025, the company holds $1.1 billion in cash and equivalents, bolstered by a $500 million financing in Q1 2025 as reported in financial statements. While R&D expenses rose to $109.8 million in Q3 2025, Beam's cash reserves are expected to sustain operations through 2028 as detailed in investor updates.
CRISPR Therapeutics and Intellia, meanwhile, face revenue pressures. CRISPR's reliance on Vertex Pharmaceuticals for Casgevy's commercialization limits its direct market exposure, while Intellia's in vivo programs require significant investment in LNP manufacturing. Beam's focus on ex vivo therapies, though capital-intensive, aligns with its current financial runway and partnerships, such as its collaboration with Apellis Pharmaceuticals to develop base-editing therapies targeting the complement system as announced.
Strategic Partnerships and Long-Term Value Creation
Beam's strategy emphasizes strategic prioritization over broad collaboration. In 2023, the company streamlined its portfolio to focus on high-value programs like BEAM-101 and BEAM-302, while exploring partnerships for select programs. This approach contrasts with CRISPR Therapeutics' alliance with Vertex and Intellia's collaboration with Regeneron, which have expanded their pipelines but diluted control over commercialization.
However, Beam's limited partnerships could pose a risk. Unlike Intellia's LNP platform or CRISPR's allogeneic CAR-T programs, BeamBEAM-- has not disclosed major collaborations for in vivo delivery or manufacturing scale-up. To create long-term value, the company must either secure strategic alliances or demonstrate that its ex vivo base-editing approach is cost-effective enough to compete with in vivo alternatives.
Conclusion: A Precision-Driven Future
Beam Therapeutics' base-editing pipeline offers a compelling case for long-term value creation in precision genetic medicine. Its clinical progress in SCD, combined with a safety profile that differentiates it from CRISPR-Cas9 and in vivo approaches, positions it to capture a significant share of the growing gene-editing market. While CRISPR Therapeutics and Intellia have first-mover advantages, Beam's focus on precision and its robust financial position provide a strong foundation for outperforming in the long run-provided it can scale manufacturing and secure partnerships to address delivery challenges.
For investors, the key question is whether the market will reward Beam's precision-driven approach with premium valuations, even as it lags in commercial approvals. The answer may hinge on the broader industry's shift toward safer, more precise editing technologies-a trend that Beam is uniquely positioned to lead.

Comentarios
Aún no hay comentarios