ATYR's Strategic Path Forward Post-Phase 3 Miss
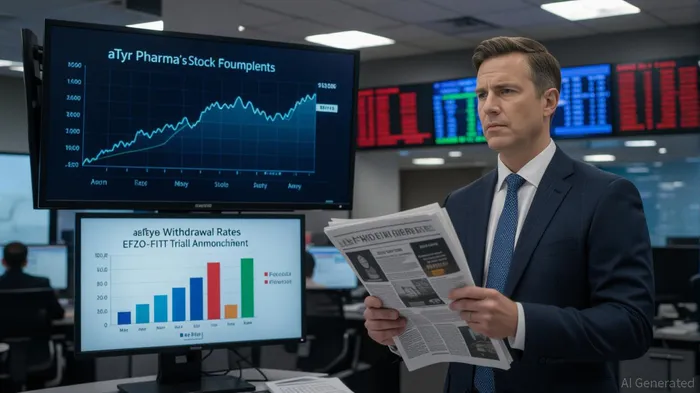
In September 2025, aTyr PharmaATYR-- faced a seismic shift in its trajectory when its Phase 3 EFZO-FIT™ trial for efzofitimod in pulmonary sarcoidosis failed to meet its primary endpoint of reducing mean daily oral corticosteroid (OCS) dose at week 48 [1]. The stock price plummeted over 80% in the aftermath [2], a stark reminder of the volatility inherent in high-risk biotech plays. Yet, the story does not end here. The trial revealed clinically meaningful secondary endpoints—52.6% of patients on the 5.0 mg/kg dose achieved complete steroid withdrawal compared to 40.2% on placebo (p=0.0919), and quality of life metrics like the King's Sarcoidosis Questionnaire (KSQ)-Lung score showed statistically significant improvements (p=0.0479) [1]. This raises a critical question: Can aTyrATYR-- leverage these data to secure regulatory approval, or must it pivot to alternative value drivers?
Regulatory Engagement: A High-Stakes Gamble
The FDA has a history of approving drugs that miss primary endpoints but demonstrate robust secondary outcomes, particularly in rare diseases. Between 2018 and 2021, 10% of newly approved drugs fell into this category [3]. For example, Carvedilol and Aricept were approved despite primary endpoint failures, relying on secondary endpoints and real-world evidence [4]. aTyr's case aligns with this precedent: efzofitimod's steroid withdrawal rates and quality of life improvements, while not statistically significant at the primary endpoint, suggest meaningful clinical benefit. The company's CEO emphasized that these outcomes could justify a “chronic, maintenance therapy” label [5], a position that may resonate with regulators given the lack of approved treatments for pulmonary sarcoidosis.
However, the path is fraught. The FDA's final guidance on multiple endpoints stresses the need for rigorous statistical controls to avoid false conclusions [6]. aTyr must convince regulators that its secondary endpoints—particularly the composite responder analysis (29.5% of efzofitimod patients achieving both steroid withdrawal and improved KSQ-Lung scores vs. 14.4% on placebo, p=0.0199) [1]—are sufficiently robust. This will require transparent dialogue and potentially additional data, such as long-term safety or real-world evidence.
Financial Resilience and Capital Raising
Despite the stock collapse, aTyr has maintained financial flexibility. In mid-2025, the company secured $30.7 million through an ATM offering, extending its operational runway beyond the Phase 3 readout [7]. This capital cushion provides breathing room to engage with the FDA and advance its pipeline. However, the reliance on a single asset—efzofitimod—remains a vulnerability. The stock's freefall underscores the risks of overexposure to one program, a cautionary tale for investors.
Alternative Value Drivers: Diversification and Partnerships
With efzofitimod's future uncertain, aTyr must explore alternative avenues. One promising avenue is its ATYR0101 program, an anti-fibrotic candidate targeting LTBP-1, which is on track for an IND filing in late 2026 [7]. Fibrosis is a $10 billion market, and aTyr's novel mechanism could attract partnerships or licensing deals. Additionally, efzofitimod's mechanism—modulating the innate immune system—might find traction in secondary indications, such as other granulomatous diseases or autoimmune conditions.
Another option is asset sales or spin-offs. While efzofitimod's Phase 3 data are mixed, its differentiated profile could still interest specialty pharma players seeking to fill unmet needs in rare diseases. The recent $1.2 billion acquisition of another sarcoidosis-focused biotech by a major player highlights the sector's potential [8].
Conclusion: A High-Risk, High-Reward Proposition
aTyr's post-EFZO-FIT strategy hinges on two pillars: persuading the FDA to accept secondary endpoints as a basis for approval or pivoting to alternative value drivers. The former is plausible but uncertain, given the agency's emphasis on statistical rigor. The latter offers long-term potential but requires patience. For investors, the key is to balance optimism with caution. aTyr's financial runway and pipeline diversification efforts provide some insulation, but the stock remains a speculative bet. In a sector where 90% of biotech assets fail in late-stage trials, aTyr's story is a reminder that even partial successes can carve out niche value—if the company navigates its next steps with precision.

Comentarios
Aún no hay comentarios