Astrazeneca's Resolute Phase III Trial Update: Assessing the Implications for Pipeline and Equity Valuation
AstraZeneca's recent update on the RESOLUTE Phase III trial for Fasenra (benralizumab) in chronic obstructive pulmonary disease (COPD) has sparked significant investor scrutiny. While the trial failed to achieve statistical significance in its primary endpoint, the drug demonstrated a consistent safety profile and numerical improvements in key metrics[1]. This outcome, coupled with broader market dynamics, raises critical questions about the drug's role in AstraZeneca's respiratory and immunology (R&I) portfolio and its long-term equity valuation.
Trial Results and Strategic Implications
The RESOLUTE trial evaluated Fasenra in moderate to very severe COPD patients with elevated eosinophil counts, a subgroup where the drug's mechanism of action—targeting interleukin-5 (IL-5) to reduce eosinophilic inflammation—was expected to yield benefits[1]. Despite the lack of statistical significance, the numerical improvement observed suggests a potential therapeutic signal in a complex, heterogeneous disease. Sharon Barr, AstraZeneca's Executive Vice President of BioPharmaceuticals R&D, emphasized the company's commitment to exploring alternative approaches, including biomarker-driven patient stratification[1].
This adaptive strategy aligns with AstraZeneca's broader R&D philosophy, which prioritizes precision medicine. For instance, Fasenra's existing approval in severe eosinophilic asthma underscores its efficacy in well-defined patient populations. The company's focus on identifying the right COPD subset—likely those with persistent eosinophilic inflammation—could mitigate the trial's immediate setback[1]. However, the failure to meet the primary endpoint may delay regulatory pathways and necessitate additional trials, potentially extending timelines for market expansion.
Equity Valuation and Market Reaction
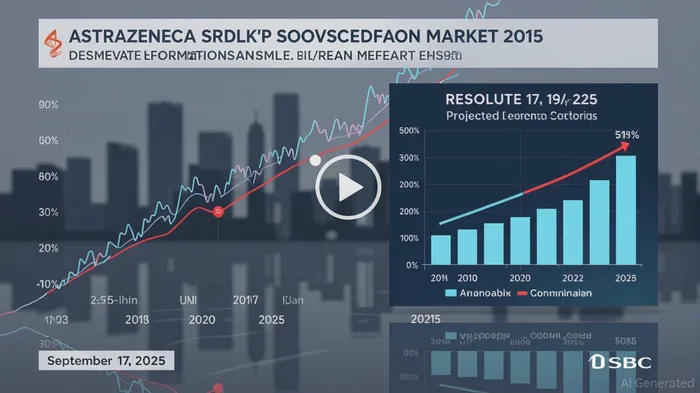
The announcement triggered a 3% decline in AstraZeneca's stock price, reflecting investor concerns over Fasenra's COPD potential[4]. This reaction, while significant, must be contextualized within the company's robust financial performance. Q1 2025 results highlighted strong revenue growth ($56.5 billion) and core earnings per share (EPS) of $5.31, with the R&I portfolio projected to account for 50% of total revenue by 2030[2]. AstraZeneca's trailing P/E ratio of 14.61 and forward P/E of 16.03 suggest a valuation anchored in earnings stability rather than speculative growth[3].
Analysts project that Fasenra could generate $1.28 billion in U.S. COPD sales by 2033, assuming regulatory approval and successful patient stratification[3]. This figure represents a meaningful contribution to the R&I portfolio, particularly as the COPD market evolves toward biologics. However, challenges such as the $35 inhaler price cap in the U.S. and competition from first-in-class therapies like Dupixent could constrain margins[3].
Long-Term Pipeline Resilience
AstraZeneca's pipeline remains a cornerstone of its long-term value proposition. Beyond Fasenra, the company's R&I portfolio includes Breztri Aerosphere, a triple-combination inhaler that has already demonstrated strong uptake in COPD and asthma-COPD overlap syndrome (ACOS)[3]. The RESOLUTE trial's limitations do not overshadow the broader momentum in respiratory and immunology, where AstraZenecaAZN-- is positioned to capture market share through innovation.
Moreover, the company's financial metrics—19.67% ROE and 11.88% ROIC—highlight efficient capital allocation and profitability[3]. These strengths provide a buffer against short-term setbacks and enable continued investment in high-potential assets. The RESOLUTE trial's mixed results may even accelerate focus on biomarker-driven trials, aligning with industry trends toward personalized medicine.
Conclusion
While the RESOLUTE trial's outcome is a near-term headwind, AstraZeneca's strategic agility and financial resilience position it to navigate the challenges ahead. The company's emphasis on precision medicine, combined with a robust R&I portfolio, ensures that Fasenra's COPD ambitions remain a long-term growth driver. Investors should monitor upcoming data analyses and regulatory updates, but the broader equity valuation appears supported by AstraZeneca's diversified pipeline and operational excellence.

Comentarios
Aún no hay comentarios