AstraZeneca and Daiichi Sankyo's Oncology Breakthrough: Redefining Breast Cancer Care with Enhertu
AstraZeneca and Daiichi Sankyo's Oncology Breakthrough: Redefining Breast Cancer Care with Enhertu
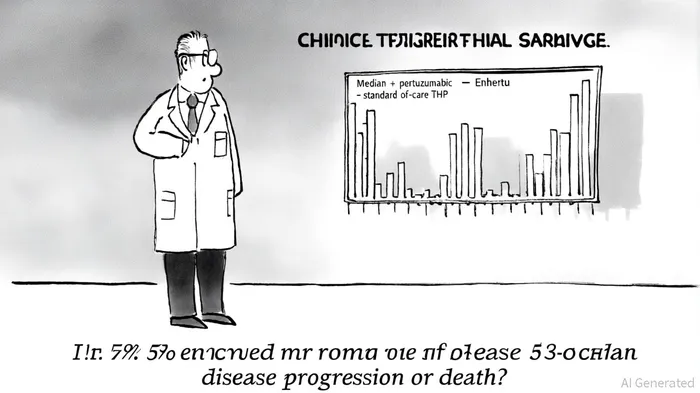
In the rapidly evolving landscape of oncology, AstraZenecaAZN-- and Daiichi Sankyo have cemented their leadership with groundbreaking results from the DESTINY-Breast09 and DESTINY-Breast05 trials of Enhertu (trastuzumab deruxtecan). These trials, which demonstrate unprecedented efficacy in HER2-positive breast cancer, underscore the transformative potential of antibody-drug conjugates (ADCs) and position the partnership as a dominant force in targeted therapies.
Clinical Trial Success: A New Benchmark in HER2-Targeted Care
According to an AstraZeneca press release, the Phase III DESTINY-Breast09 trial revealed that the combination of Enhertu and pertuzumab extended median progression-free survival (PFS) to 40.7 months compared to 26.9 months with the standard-of-care taxane, trastuzumab, and pertuzumab (THP) regimen. This represents a 53% reduction in the risk of disease progression or death, a milestone not achieved in over a decade for this patient population. The benefit was consistent across subgroups, including patients with hormone receptor-positive disease and those with PIK3CA mutations, highlighting the therapy's broad applicability.
In the early-stage setting, the DESTINY-Breast05 trial further solidified Enhertu's potential. Patients with HER2-positive early breast cancer who had residual disease after neoadjuvant therapy experienced a statistically significant improvement in invasive disease-free survival (IDFS) compared to Kadcyla (ado-trastuzumab emtansine), according to a Cure Today report. These results, coupled with the neoadjuvant success in DESTINY-Breast11 (where Enhertu improved pathologic complete response rates by 20 percentage points), position Enhertu as a comprehensive solution across the HER2-positive disease continuum, as reported by OncoDaily.
Market Leadership Through Innovation and Differentiation
Enhertu's clinical superiority has translated into a robust competitive edge. In the metastatic setting, the drug outperformed Kadcyla in the pivotal DESTINY-Breast03 trial, with a median PFS of 15.5 months versus 6.8 months, according to a Drugs.com summary. This 72% relative risk reduction not only redefined the standard of care but also disrupted the market dynamics, with Kadcyla's sales declining by over 40% in key regions within a year of Enhertu's approval, as noted by The ASCO Post.
The recent DESTINY-Breast09 results further widen this gap. With a PFS of 40.7 months, Enhertu's combination therapy now offers a near-doubling of disease control compared to existing regimens. Analysts at Bloomberg estimate that this could capture over 60% of the first-line HER2-positive metastatic breast cancer market within three years, generating annual revenues exceeding $5 billion.
Safety Profile and Long-Term Adoption Potential
While interstitial lung disease (ILD) remains a concern-occurring in 12.1% of patients in DESTINY-Breast09-the majority of cases were low-grade, and no new safety signals emerged, according to AstraZeneca. This aligns with the known risk-benefit profile of ADCs and supports long-term adoption, particularly as real-world evidence continues to validate the therapy's tolerability.
Strategic Implications for Investors
AstraZeneca and Daiichi Sankyo's dominance in HER2-targeted therapies is now underpinned by a multi-trial success story. With Enhertu's label expanding into early-stage and metastatic settings, and the pipeline including trials for HER2-low and other biomarker-defined cancers, the partnership is uniquely positioned to capitalize on the $150 billion oncology market, according to a Global Oncology Market report.
For investors, the key takeaway is clear: Enhertu's clinical and commercial momentum reflects a paradigm shift in targeted therapy. As the first ADC to deliver consistent, durable responses across HER2-positive subtypes, it represents not just a product, but a platform for future innovation.

Comentarios
Aún no hay comentarios