Assessing the Impact of IPR&D Charges on BioMarin's Value Creation and R&D Pipeline Momentum
Assessing the Impact of IPR&D Charges on BioMarin's Value Creation and R&D Pipeline Momentum
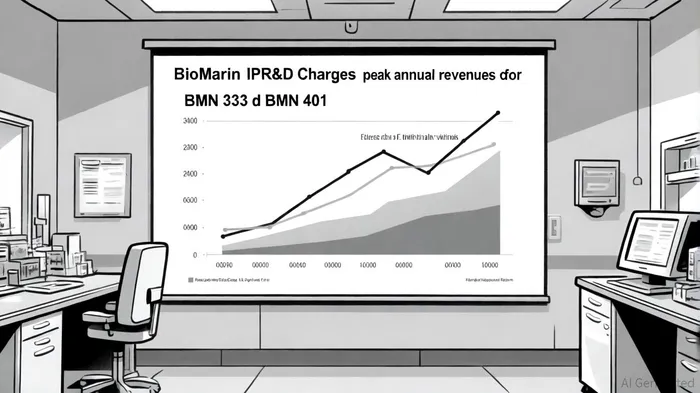
BioMarin Pharmaceutical Inc. (BMRN) faces a critical juncture in 2025 as it navigates the financial and strategic implications of its $221 million in-process research and development (IPR&D) charge from the Inozyme acquisition. This charge, set to hit third-quarter 2025 results, represents a significant near-term drag on earnings but is framed by the company as a calculated investment in long-term value creation through its expanding rare disease pipeline. The challenge for investors lies in balancing the immediate costs against the potential future returns from assets like BMN 333 and BMN 401, which could redefine BioMarin's revenue trajectory.
The IPR&D Charge: A Strategic Trade-Off
BioMarin's Q3 2025 IPR&D charge of $221 million-equivalent to a $1.10 per share hit to both GAAP and Non-GAAP diluted EPS-stems from the July 2025 acquisition of Inozyme, a developer of therapies for ultra-rare metabolic disorders, according to an Investing.com report. While such charges are non-cash and non-recurring, they underscore the high-stakes nature of biotech M&A, where the value of unproven assets must be weighed against their potential. BioMarin's management has emphasized that these charges are "not typically forecasted" due to their inherent uncertainty, and the Investing.com report noted this uncertainty, yet the company reaffirmed its full-year 2025 guidance, signaling confidence in its ability to offset the short-term pain with long-term gains.
Pipeline Momentum: BMN 333 and BMN 401 as Growth Catalysts
The Inozyme acquisition added two late-stage assets to BioMarin's portfolio: BMN 333, a long-acting C-type natriuretic peptide (CNP) for achondroplasia, and BMN 401, a first-in-disease enzyme replacement therapy for ENPP1 Deficiency. Both programs represent high-conviction bets on genetically defined rare diseases, a strategic sweet spot for BioMarinBMRN--.
BMN 333 has already demonstrated superior pharmacokinetic (PK) profiles in Phase 1 trials, with area-under-the-curve (AUC) levels three times higher than competing CNPs, according to a BioMarin press release. This positions it to potentially outperform BioMarin's existing achondroplasia therapy, Voxzogo, and capture a significant share of a market projected to grow from $185.69 million in 2025 to $294.01 million by 2030 at a 9.69% CAGR. A pivotal Phase 2/3 trial is slated for H1 2026, with a potential 2030 launch. Analysts, however, caution that revenue from BMN 333 is unlikely before 2030 (the Investing.com report highlights this timing), creating a multi-year gap between the IPR&D charge and tangible returns.
BMN 401, targeting ENPP1 Deficiency, is further along in development, with pivotal data from the ENERGY 3 study in children expected in H1 2026 and a potential 2027 launch, as noted in an Inozyme presentation. ENPP1 Deficiency affects approximately 10,000 patients globally, with no approved therapies, creating a $1 billion addressable market. Inozyme's internal projections suggest BMN 401 could achieve peak annual sales of $400–600 million by the mid-2030s, making it a high-impact asset despite its small patient base.
Balancing Near-Term Costs and Long-Term Rewards
The key question for investors is whether BioMarin's current financial health justifies these strategic bets. The company's Q2 2025 results-$825 million in revenue (+16% YoY) and non-GAAP EPS of $1.44 (+50% YoY)-demonstrate robust cash flow from existing therapies like Voxzogo and PALYNZIQ, as reported in its press release. This financial flexibility allows BioMarin to absorb the IPR&D charge while funding pipeline advancements. However, the time lag between the $221 million expense and the projected returns from BMN 333 and BMN 401 introduces execution risk. Delays in trials or regulatory hurdles could erode investor confidence, particularly if the company fails to meet its 2030 revenue targets.
Strategic Rationale and Market Positioning
BioMarin's acquisition of Inozyme aligns with its long-term strategy of dominating high-impact rare disease markets. By adding BMN 401 and BMN 333 to its portfolio, the company is diversifying its revenue streams and extending its leadership in enzyme therapies. The ENPP1 Deficiency market, in particular, offers a unique opportunity to capture premium pricing due to the disease's severity and lack of alternatives. Meanwhile, BMN 333's potential to become a best-in-class CNP could solidify BioMarin's position in achondroplasia, a therapeutic area with growing demand as awareness and reimbursement expand.
Conclusion: A Calculated Bet on the Future
BioMarin's IPR&D charge in Q3 2025 is a necessary short-term cost to secure long-term value creation. While the $221 million hit may test investor patience, the company's strong current performance and the high unmet need addressed by BMN 333 and BMN 401 justify the strategic investment. Success in these programs could transform BioMarin into a leader in two ultra-rare disease markets, generating billions in peak annual revenue. However, the path to profitability remains contingent on clinical and regulatory execution-factors that will define whether this calculated bet pays off.

Comentarios
Aún no hay comentarios