The Asia-Pacific Liquid Biopsy Market: Strategic Advantages for Early-Movers in Non-Invasive Cancer Diagnostics
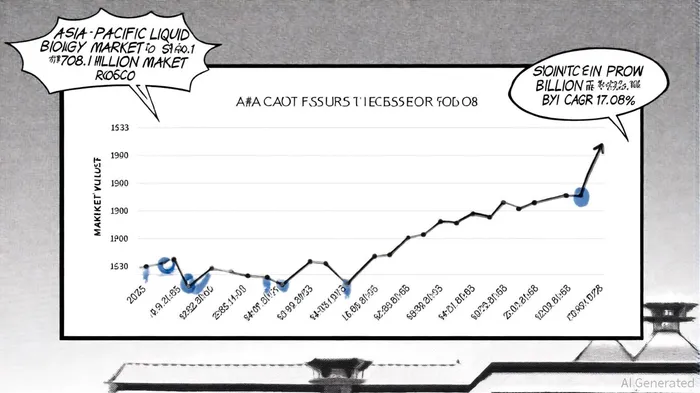
The Asia-Pacific liquid biopsy market is emerging as a cornerstone of modern oncology, driven by its non-invasive approach to cancer detection and monitoring. By 2024, the market was valued at $708.1 million, and it is projected to surge to $2.92 billion by 2033, growing at a compound annual growth rate (CAGR) of 17.08%, according to a GlobeNewswire report. This expansion is fueled by rising cancer prevalence, advancements in biomarker detection, and the integration of artificial intelligence (AI) and next-generation sequencing (NGS) technologies, per a Grand View Research report. For investors, early-mover companies in this space are leveraging strategic partnerships, regulatory approvals, and technological innovation to secure a dominant position in a rapidly evolving landscape.
Market Dynamics and Growth Drivers
The Asia-Pacific region's liquid biopsy market is uniquely positioned to capitalize on global trends in precision medicine. According to a GlobeNewswire report, the market's growth is attributed to the increasing adoption of minimally invasive diagnostics, which reduce patient discomfort and enable real-time monitoring of cancer progression. Additionally, government initiatives promoting genomic research and the shift toward personalized treatment plans are accelerating adoption. For instance, Singapore's Health Sciences Authority approved Guardant360 CDx in 2023 for non-small cell lung cancer (NSCLC) and comprehensive genomic profiling, a move that has set a regulatory precedent for other countries in the region, according to Biotech Connection.
Strategic Advantages of Early-Movers
Early adopters in the Asia-Pacific liquid biopsy market are distinguishing themselves through innovative partnerships and technological differentiation.
- A.D.A.M. Innovations and SOPHiA GENETICS: This Japanese collaboration commercialized a liquid biopsy companion diagnostic (CDx) test that leverages SOPHiA GENETICS' AI-powered SOPHiA DDM™ Platform to detect actionable genomic alterations from a single blood draw. The partnership reduces turnaround times and costs, addressing key barriers to adoption, as reported by Taiwan News.
- BioNexus Gene Lab Corp. and Fidelion Diagnostics: In Southeast Asia, this partnership introduced VitaGuard™ MRD, a platform that detects cancer recurrence through blood samples at under $300 per test, significantly undercutting traditional methods, according to QuiverQuant.
- Prenetics Group and Chinese University of Hong Kong: A joint venture to develop a multi-cancer early detection (MCED) test for liver and lung cancers, slated for a 2025 launch, highlights the region's focus on scalable solutions, per a Business Wire release.
These examples underscore how early-movers are combining local expertise with global technology to address unmet clinical needs. For instance, Singapore-based Lucence and Mirxes have developed region-specific tests like LiquidHallmark (ctDNA profiling) and GASTROClear (early-stage gastric cancer detection), catering to the unique epidemiological profile of Asia-Pacific populations, as noted by Biotech Connection.
Technological and Regulatory Tailwinds
The integration of NGS and AI is a critical differentiator for market leaders. NGS dominates the technology segment in 2024, enabling multi-gene-parallel analysis for higher sensitivity, according to the Grand View Research report. Meanwhile, AI enhances diagnostic accuracy by identifying genomic alterations with precision, as seen in Guardant Health's assays and described by Straits Research.
Regulatory frameworks are also maturing. Japan and Singapore have established clear pathways for liquid biopsy approvals, while countries like China are investing heavily in genomic research infrastructure, per the GlobeNewswire report. These developments reduce time-to-market for new products and provide early-movers with a competitive edge.
Challenges and Mitigation Strategies
Despite its promise, the market faces hurdles. Technical limitations, such as the potential for undetected mutations in early-stage cancers, persist, a point highlighted by Biotech Connection. Reimbursement policies for NGS-based tests remain inconsistent across the region, limiting accessibility, as noted in the GlobeNewswire analysis. However, companies are addressing these challenges through collaborative R&D and cost optimization. For example, VitaGuard's affordability model and SOPHiA GENETICS' AI-driven efficiency are setting benchmarks for scalability.
Conclusion: A Lucrative Opportunity for Investors
The Asia-Pacific liquid biopsy market offers a compelling investment thesis for those who recognize the strategic advantages of early-movers. Companies that combine technological innovation, regulatory agility, and cost-effective solutions are poised to dominate this high-growth sector. As the market matures, partnerships with academic institutions and AI-driven advancements will further solidify their positions. For investors, the key lies in identifying firms that not only address technical challenges but also align with the region's unique healthcare needs.

Comentarios
Aún no hay comentarios