Arcus Bioscience's Phase 2 Edge-Gastric Trial and Its Implications for Immuno-Oncology: Assessing Strategic Investment Potential in a High-Stakes Era
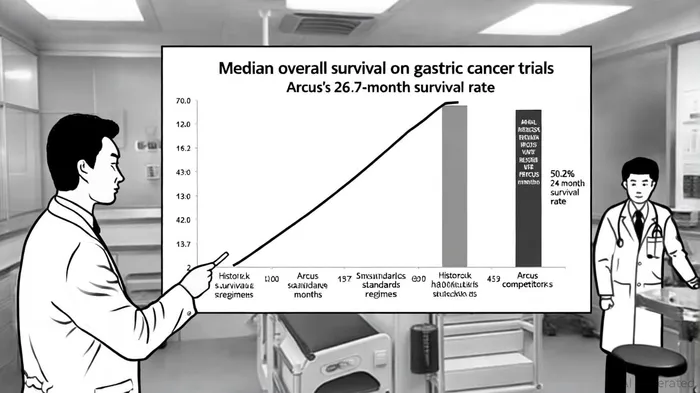
Arcus Biosciences has emerged as a focal point in the immuno-oncology (IO) landscape following the presentation of groundbreaking data from its Phase 2 EDGE-Gastric trial at the 2025 European Society for Medical Oncology (ESMO) Congress. The trial evaluated a combination of domvanalimab (an Fc-silent anti-TIGIT antibody), zimberelimab (an anti-PD-1 monoclonal antibody), and chemotherapy in patients with advanced gastric, gastroesophageal junction, or esophageal adenocarcinoma. The results-a median overall survival (OS) of 26.7 months and 50.2% of patients surviving 24 months or longer-have redefined expectations for first-line treatment in this historically underserved cancer type, according to the Phase 2 EDGE-Gastric report. For investors, these outcomes represent not just a clinical milestone but a strategic inflection point for ArcusRCUS-- in the broader IO innovation race.
Clinical and Commercial Implications of the EDGE-Gastric Trial
The EDGE-Gastric trial's success underscores Arcus's ability to leverage its Fc-silent TIGIT inhibition platform to overcome resistance mechanisms in solid tumors. Unlike traditional checkpoint inhibitors, domvanalimab's design minimizes off-target effects while enhancing T-cell activation, a critical advantage in cancers like gastric adenocarcinoma, where immune evasion is pervasive. The regimen's sustained efficacy across all PD-L1 subgroups further differentiates it from existing therapies, which often rely on biomarker stratification to identify responders.
From a commercial standpoint, the trial's results position Arcus to challenge entrenched players in the gastric cancer market. Merck's Keytruda (pembrolizumab) and Roche's Tecentriq (atezolizumab) dominate the PD-1/PD-L1 space, but their combination with chemotherapy typically yields median OS of 10–14 months in advanced gastric cancer, according to a Global Growth Insights report. Arcus's 26.7-month median OS not only doubles this benchmark but also aligns with the growing demand for durable responses in oncology. Analysts at Mizuho Securities have highlighted that the trial's positive safety profile-consistent with standard anti-PD-1 and chemotherapy regimens-reinforces its potential for rapid adoption, according to Business Insider.
Strategic Positioning in the Immuno-Oncology Arms Race
Arcus's competitive edge extends beyond gastric cancer. The company's dual focus on TIGIT inhibition and HIF-2α inhibition (via its lead asset, casdatifan) positions it to capitalize on two of the most promising frontiers in IO. Casdatifan, currently in Phase 3 trials for clear cell renal cell carcinoma (RCC), has demonstrated an objective response rate (ORR) of 46% in combination with cabozantinib, outperforming Merck's belzutifan (ORR of 30%) in similar settings, as shown in the Arcus Q2 2025 slides. This dual-therapy approach, coupled with Arcus's financial runway of $1 billion, provides a robust foundation for navigating the high-risk, high-reward environment of oncology R&D (the same Arcus Q2 2025 slides outline the company's cash position).
However, the path to commercialization is not without hurdles. TIGIT inhibitors face intense competition from Bristol-Myers Squibb's tiragolumab and Merck's MK-7684, both of which are in late-stage trials for non-small cell lung cancer (NSCLC) and other indications, per a recent SWOT analysis. Arcus's differentiation lies in its Fc-silent design, which reduces the risk of immune-related adverse events, and its broad pipeline spanning gastric, lung, and renal cancers. The upcoming Phase 3 STAR-221 trial, which will evaluate the EDGE-Gastric regimen in a larger cohort, will be pivotal in validating these advantages (see Phase 2 EDGE-Gastric report).
Market Reaction and Investment Thesis
Despite a 11.8% year-to-date decline in its stock price, Arcus has attracted renewed investor interest. Over the past month, its shares have surged 9.5%, driven by positive trial data and a $21.14 average price target from Wall Street analysts, implying an 81.67% upside, according to the MarketBeat price target. This optimism is further fueled by Arcus's expansion into autoimmune and inflammatory diseases, a move that diversifies its revenue streams and mitigates reliance on oncology alone (see SWOT analysis).
For strategic investors, the key question is whether Arcus can translate its Phase 2 success into regulatory approval and market share. The EDGE-Gastric results provide a compelling case for the latter, particularly given the lack of effective first-line therapies in gastric cancer. However, the company's valuation remains anchored to its ability to replicate these outcomes in Phase 3 and secure partnerships for global commercialization.
Conclusion: A High-Risk, High-Reward Proposition
Arcus Biosciences stands at a crossroads in the immuno-oncology revolution. The EDGE-Gastric trial has demonstrated the transformative potential of its TIGIT inhibition platform, while its HIF-2α inhibitor pipeline offers a complementary avenue for growth. Yet, the company's success hinges on its ability to navigate a crowded competitive landscape and deliver consistent data across its late-stage trials. For investors with a long-term horizon and a tolerance for volatility, Arcus represents a compelling bet on the future of cancer immunotherapy-a sector poised to grow at 19.19% CAGR through 2033 (see Global Growth Insights report).

Comentarios
Aún no hay comentarios