Apimeds' Strategic Positioning in the Non-Opioid Pain Management Sector: Regulatory Catalysts and Market Readiness
The non-opioid pain management sector is undergoing a transformative phase, driven by regulatory tailwinds and a market increasingly primed for alternatives to opioid-based therapies. For companies like Apimeds PharmaceuticalsAPUS--, these dynamics present both challenges and opportunities. With its lead candidate, Apitox, in late-stage development for chronic osteoarthritis pain, ApimedsAPUS-- is strategically aligning itself with industry trends that prioritize safer, non-opioid solutions.
Regulatory Catalysts: FDA Guidance and Fast-Track Pathways
A pivotal development in 2025 was the U.S. Food and Drug Administration's (FDA) release of its draft guidance on the development of non-opioid analgesics for chronic pain[1]. This guidance streamlines regulatory pathways by allowing a single, well-controlled chronic pain trial supported by confirmatory evidence, potentially accelerating approvals for products like Apitox[1]. Apimeds has explicitly positioned itself to leverage this framework, with CEO statements highlighting the potential for fast-track or breakthrough designations[1]. Such designations could significantly reduce development timelines and costs, a critical advantage in a competitive landscape where Vertex PharmaceuticalsVRTX-- recently secured FDA approval for Journavx, the first non-opioid analgesic for acute pain[2].
The FDA's broader strategy to curb opioid misuse—ranging from updated safety labeling to enhanced enforcement against illicit opioid imports—further underscores the regulatory momentum favoring non-opioid alternatives[1]. For Apimeds, this creates a dual benefit: reduced regulatory uncertainty and a clearer path to market differentiation.
Market Readiness: Growth Projections and Payment Model Shifts
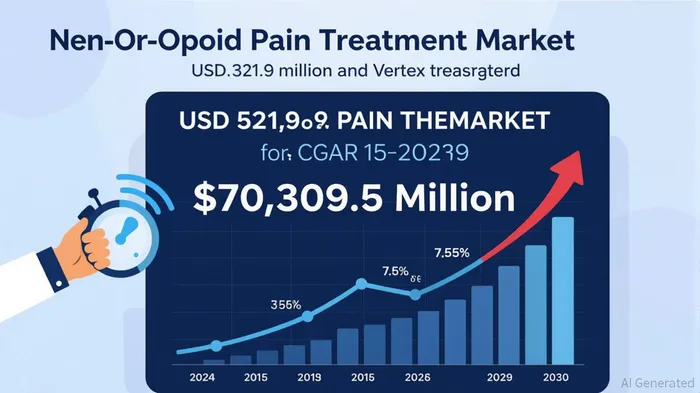
The global non-opioid pain treatment market, valued at USD 45,321.9 million in 2024, is projected to reach USD 70,309.5 million by 2030, growing at a compound annual rate of 7.7%[1]. This expansion is fueled by rising surgical volumes, heightened awareness of opioid risks, and evolving payment models. By 2020, nearly 15% of value-based care arrangements had transitioned to advanced alternative payment models (APMs), including shared savings programs that incentivize providers to adopt non-opioid therapies[1]. These models are now extending into behavioral health and substance use disorder treatment, signaling a systemic shift toward holistic, non-opioid care.
Medicare's inclusion of ZYNRELEF in its 2025 Non-Opioid Pain Relief Policy[1] further illustrates payer alignment with this trend. As reimbursement structures evolve to reward outcomes over volume, companies with robust non-opioid portfolios—like Apimeds—are poised to capture market share.
Apimeds' Strategic Moves: Innovation and Collaboration
Beyond regulatory and market trends, Apimeds has taken proactive steps to strengthen its position. Its partnership with the University of Alabama's Culverhouse College of Business to launch the ai² Future Labs program[2] is a strategic move to tap into academic expertise for drug development and commercialization planning. This initiative not only addresses the technical challenges of bringing Apitox to market but also cultivates a pipeline of talent familiar with the nuances of non-opioid innovation.
The company's focus on chronic osteoarthritis pain—a condition affecting over 30 million Americans[2]—is also well-aligned with unmet medical needs. While NSAIDs currently dominate the market[1], their long-term use is associated with gastrointestinal and cardiovascular risks, creating a gap for safer alternatives like Apitox.
Risks and Considerations
Despite these positives, challenges remain. Smaller providers, in particular, struggle to integrate value-based care models into their workflows[1], and Apimeds' success will depend on its ability to demonstrate Apitox's cost-effectiveness in real-world settings. Additionally, competition is intensifying: Tris Pharma's cebranopadol, a dual-NMR agonist, recently reported positive phase 3 results[2], and Vertex's Journavx has already secured a foothold in acute pain management[2].
Conclusion: A Position of Strength
Apimeds' strategic alignment with regulatory catalysts and market readiness positions it as a compelling player in the non-opioid pain management sector. By capitalizing on the FDA's streamlined pathways, leveraging academic partnerships, and targeting a high-need therapeutic area, the company is well-placed to navigate the sector's evolving landscape. As the market grows and payment models continue to shift, Apimeds' ability to deliver a differentiated, non-opioid solution could translate into significant long-term value.

Comentarios
Aún no hay comentarios