Amylyx Pharmaceuticals' Recent Equity Offering: Strategic Funding for Avexitide Commercialization and R&D Momentum
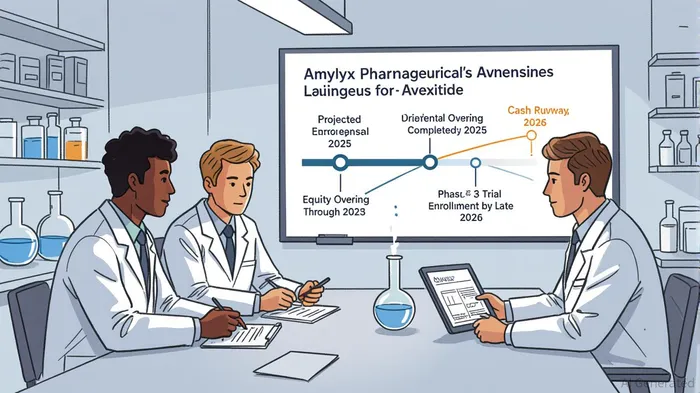
Amylyx Pharmaceuticals' recent equity offering represents a calculated strategic move to accelerate the commercialization of Avexitide, its lead candidate for post-bariatric hypoglycemia (PBH), while sustaining R&D momentum across its pipeline. The offering, which includes a 15% over-allotment option for underwriters, underscores the company's focus on securing capital to navigate critical near-term milestones, including the completion of the Phase 3 LUCIDITY trial and preparation for a potential 2027 market launch[1].
Strategic Allocation of Funds: Balancing Commercial Readiness and R&D
The net proceeds from the offering will be directed toward three pillars: Avexitide commercial readiness (estimated 40%), research and development (30%), and general corporate purposes (30%)[2]. This allocation reflects Amylyx's dual imperative to build infrastructure for a first-in-class therapy while advancing its broader pipeline, including AMX0035 for neurodegenerative diseases.
The emphasis on commercial readiness is particularly noteworthy. With no approved treatments for PBH—a condition affecting ~160,000 U.S. patients annually—Amylyx aims to establish a robust commercial foundation. This includes hiring Dan Monahan, a seasoned executive with expertise in launching therapies for niche markets, and investing in market education and payer access strategies[3]. Such efforts are critical to securing reimbursement and physician adoption, given the complexity of PBH management and the absence of existing benchmarks.
R&D funding will support ongoing trials, including the Phase 3 LUCIDITY study, where Avexitide has demonstrated a 64% reduction in hypoglycemic events in earlier trials[4]. The FDA's agreement on the primary endpoint—reducing composite Level 2 and Level 3 hypoglycemic events over 16 weeks—positions the trial for a clear regulatory pathway[5].
Risk Mitigation: Cash Runway and Burn Rate Optimization
Amylyx's financial prudence is evident in its reduced cash burn rate. Q2 2025 expenses fell to $41.4 million, a 43% decline from $72.7 million in Q2 2024, driven by a 28% reduction in SG&A costs[6]. Combined with $180.8 million in cash reserves as of June 30, 2025, and the $65.5 million raised in a January 2025 offering, the company now has a runway extending into 2026[7]. This timeline aligns with the expected completion of the LUCIDITY trial, reducing the need for near-term dilutive financing and mitigating liquidity risk.
However, the offering's success hinges on market conditions and the execution of its commercial strategy. The 15% over-allotment option provides flexibility but could dilute existing shareholders if exercised. Investors must weigh this against the potential for Avexitide to capture a significant share of the PBH market, which is currently underserved and lacks competitive threats in late-stage development[8].
Competitive Landscape and Market Potential
Avexitide's position as the only Phase 3-ready GLP-1 receptor antagonist for PBH strengthens its commercial outlook. While companies like Vogenx and MBX BiosciencesMBX-- are exploring alternative therapies (e.g., mizagliflozin, long-acting GLP-1 antagonists), none have matched Avexitide's regulatory momentum or clinical efficacy profile[9]. The drug's Breakthrough Therapy and Orphan Drug designations further enhance its appeal to payers and regulators, potentially accelerating approval timelines.
Amylyx's partnerships, including a collaboration with Gubra to develop a long-acting GLP-1 antagonist, also signal a commitment to addressing unmet needs in PBH beyond Avexitide's initial launch[10]. These efforts could create a durable market presence, particularly if Avexitide's safety and efficacy data in 2026 reinforce its value proposition.
Risk-Adjusted Returns: A Calculated Bet
The offering's strategic value lies in its alignment with Amylyx's near-term milestones. A successful Phase 3 trial and subsequent approval would unlock a high-margin, niche market with strong pricing potential. However, risks remain: trial failure, regulatory delays, or inadequate commercial execution could undermine returns. The reduced cash burn rate and extended runway provide a buffer, but investors must monitor enrollment progress in LUCIDITY and the company's ability to secure partnerships or co-promotion deals.
In conclusion, Amylyx's equity offering is a well-timed capital raise that addresses both immediate operational needs and long-term growth. By prioritizing commercial readiness and leveraging its clinical lead, the company is positioning Avexitide to become a transformative therapy for PBH—a market with significant unmet demand and limited competition. For investors, the offering represents a high-risk, high-reward opportunity contingent on the successful execution of its clinical and commercial strategy.

Comentarios
Aún no hay comentarios