Accelerating Arcutis: FDA Approvals and Pediatric Dermatology Demand Fuel a New Growth Paradigm
The biotechnology sector has long been defined by the interplay between regulatory milestones and market dynamics. In 2025, ArcutisARQT-- Biotherapeutics has emerged as a case study in how strategic FDA approvals can catalyze both therapeutic innovation and commercial expansion. The recent authorization of ZORYVE® (roflumilast) cream 0.05% for pediatric atopic dermatitis (AD) in children aged 2 to 5 years, alongside its plaque psoriasis indication for adults, underscores a dual-axis growth strategy that is redefining the company's trajectory.
Market Expansion: From Niche to Mainstream
Arcutis' ZORYVE portfolio has demonstrated remarkable scalability. According to Arcutis' Q2 2025 results, Q2 2025 net product revenue for ZORYVE reached $81.5 million, a 164% increase compared to Q2 2024 (Arcutis' Q2 2025 results). This surge is driven by the product's broad therapeutic applicability-spanning plaque psoriasis, AD, and now, pediatric AD-as well as its favorable safety profile. The FDA's May 2025 approval of ZORYVE topical foam 0.3% for plaque psoriasis in patients aged 12 and older expanded treatment access to nearly 9 million Americans, according to MarketBeat's FDA approvals tracker (MarketBeat's FDA approvals tracker), while the October 2025 pediatric AD approval addressed a $1.2 billion market segment previously underserved by nonsteroidal options, as Business Insider reported (Business Insider).
The commercial infrastructure supporting ZORYVE further amplifies its market potential. All three major U.S. pharmacy benefit managers (PBMs) now cover the product, and the National Psoriasis Foundation has awarded it the Seal of Recognition, as detailed in Arcutis' Q1 2025 report (Arcutis' Q1 2025 report). These endorsements not only enhance accessibility but also signal to investors a robust value proposition in a competitive dermatology landscape.
Pediatric Dermatology: A Strategic Differentiator
The pediatric AD market represents a critical unmet need. Over 1.8 million children in the U.S. aged 2 to 5 years suffer from mild to moderate AD, yet long-term steroid use carries risks such as skin atrophy and adrenal suppression, as Healio reported (Healio). ZORYVE's phosphodiesterase-4 (PDE4) inhibition mechanism offers a steroid-free alternative, with clinical trials showing 25.4% of treated children achieving complete or near-complete clearance compared to 10.7% in placebo groups, according to Dermatology Times (Dermatology Times).
This innovation is not merely therapeutic but also behavioral. As Arcutis' press release noted, the cream's formulation-free of sensitizing agents and suitable for sensitive areas like the face-aligns with caregiver preferences for nonirritating, easy-to-apply treatments (Arcutis' press release). The rapid onset of action (significant improvement within four weeks) further strengthens its appeal in a market where chronic disease management is paramount.
Pipeline and Future Prospects
Arcutis' growth is not confined to ZORYVE. The company has submitted an Investigational New Drug (IND) application for ARQ-234, a fusion protein targeting AD, and is advancing ARQ-255-a topical suspension for alopecia areata-with pivotal results expected in early 2025, according to Quiver QuantQNT-- (Quiver Quant). These pipeline advancements, coupled with plans to expand ZORYVE's pediatric indication to infants as young as 3 months, position Arcutis to dominate multiple dermatological niches.
Conclusion: A Model for Regulatory-Driven Growth
The convergence of FDA approvals, commercial infrastructure, and unmet clinical needs has transformed Arcutis into a high-conviction investment. By addressing pediatric AD-a segment with both medical and economic urgency-and expanding into plaque psoriasis, the company has created a compounding growth engine. For investors, the key takeaway is clear: regulatory milestones are no longer just hurdles but accelerants for market leadership in an industry where innovation and accessibility are increasingly intertwined.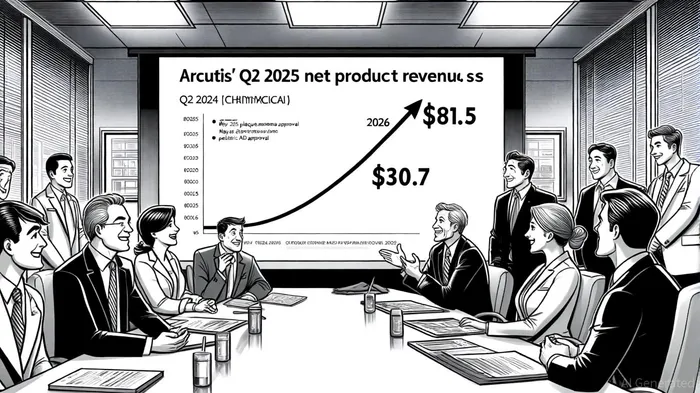

Comentarios
Aún no hay comentarios