Acadian's Growing Stake in Aclaris Therapeutics: A Catalyst for Re-Rating Amid Promising Phase 2a Data
The recent surge in institutional and insider confidence in Aclaris TherapeuticsACRS-- (NASDAQ: ACRS) has positioned the biopharma stock as a compelling case study in how clinical progress and strategic capital flows can catalyze a re-rating. At the heart of this narrative is Acadian AssetAAMI-- Management's 25.9% increase in its stake during Q1 2025, now representing a 2.03% ownership in the company[2]. This move, coupled with insider purchases and pharmacodynamic validation of ATI-2138's mechanism, suggests a convergence of clinical and financial signals that could unlock significant value.
Clinical Progress: A Differentiated Profile in a Competitive Space
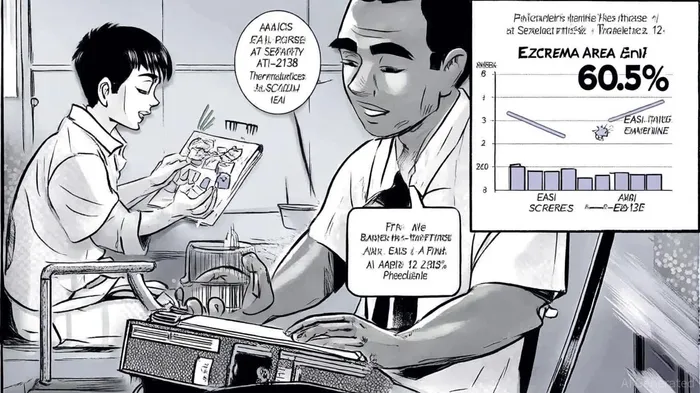
Aclaris' Phase 2a trial of ATI-2138 in moderate-to-severe atopic dermatitis (AD) delivered results that directly address the limitations of existing JAK inhibitors. The trial reported a 60.5% mean improvement in EASI scores at week 12, with rapid onset of action observed as early as week 1[1]. Notably, the drug demonstrated a favorable safety profile, with no severe adverse events (SAEs) or treatment-emergent adverse events (TEAEs) reported. This is a critical differentiator in a class where cardiovascular and metabolic risks have constrained adoption.
Pharmacodynamic data further reinforced the therapeutic potential of ITK inhibition, showing robust downregulation of inflammatory markers such as IL-13 and IL-4[1]. These findings not only validate ITK as a target but also suggest that ATI-2138 could avoid the broad immunosuppression associated with pan-JAK inhibitors. With the drug now slated for presentation at the 2025 EADV Congress in Paris, institutional credibility and investor awareness are poised to rise.
Institutional and Insider Confidence: A Synchronized Bet
Acadian's quarter-on-quarter accumulation of 451,044 shares—valued at $3.36 million—reflects a strategic bet on Aclaris' pipeline[2]. The firm's 2.03% ownership stake, combined with its historical focus on biotech innovation, underscores a calculated alignment with the company's risk-reward profile. Meanwhile, insider activity has added another layer of conviction. Anand Mehra, Aclaris' CEO, purchased 666,666 shares for $1.5 million within six months[2], signaling alignment with long-term shareholders.
Institutional investors have mirrored this optimism. ADAGE Capital Partners and VIVO Capital added over 18 million shares in late 2024, collectively investing $45.9 million[2]. These moves suggest that both insiders and external investors view Aclaris as a high-conviction play, particularly given the drug's potential to expand into indications like alopecia areata—a $3 billion market with unmet needs.
Strategic Implications: From Re-Rating to Re-Positioning
The combination of clinical differentiation and capital inflows creates a self-reinforcing dynamic. A re-rating of ACRSACRS-- would likely stem from three factors:
1. Pipeline Validation: Positive Phase 2a data in AD, a $4.5 billion market, positions ATI-2138 as a best-in-class candidate.
2. Safety Premium: The absence of SAEs could allow Aclaris to command a premium valuation over peers with riskier profiles.
3. Expansion Potential: ITK inhibition's applicability to autoimmune and inflammatory diseases opens pathways for multi-indication development.
However, risks remain. The trial's small sample size (n=14) necessitates larger Phase 2b trials to confirm results. Additionally, competition from established JAK inhibitors like Pfizer's Xeljanz and Incyte's Jakavi means Aclaris must demonstrate not just efficacy but also cost-effectiveness.
Conclusion: A Convergence of Signals
Aclaris Therapeutics stands at an inflection point. The Phase 2a data for ATI-2138, while preliminary, addresses key unmet needs in AD and positions the drug as a safer alternative to JAK inhibitors. Acadian's aggressive stake increase, insider purchases, and institutional inflows collectively signal a growing consensus that Aclaris is undervalued relative to its pipeline potential. As the company prepares for regulatory and investor scrutiny at the EADV Congress, the stage is set for a re-rating that could redefine its market position.
For investors, the question is no longer whether ATI-2138 works—but whether the market is prepared to price in its full potential.

Comentarios
Aún no hay comentarios